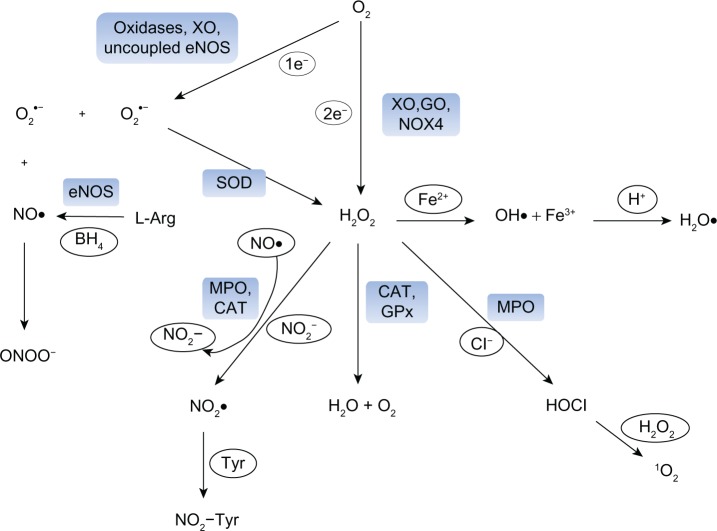
Figure 1.
Formation of reactive oxygen species (ROS) in vascular cells. The reduction of oxygen (O2) by one electron leads to the formation of superoxide anion (O2•−), which can be either dismutated to hydrogen peroxide (H2O2) spontaneously or in a reaction catalyzed by superoxide dismutase (SOD). Nitric oxide (NO•) is produced by endothelial nitric oxide synthase (eNOS) from L-arginine (L-Arg) and tetrahydrobiopterin. O2•− and NO• react spontaneously with each other to form peroxynitrite (ONOO−). H2O2 can also be generated directly from oxygen by some vascular oxidases, such as xanthine oxidase (XO), glucose oxidase (GO) and NOX4-containing NADPH-oxidases (NOX4). H2O2 can be scavenged by catalase (CAT) or glutathione peroxidase (GPx) to form water and oxygen or can undergo non-enzymatic reactions to generate the hydroxyl radical (OH•) in the metal-catalyzed Haber-Weiss or Fenton reaction. OH• may be protonated to the hydroperoxyl radical. Ferrous-containing enzymes, such as myeloperoxidase (MPO) are activated by H2O2 to form a highly reactive radical that can oxidize NO• to nitrogen dioxide anion (NO2−) and react with NO2− to form nitrogen dioxide radical (NO2•). NO2• can, in turn, participate in nitrating events, such as the formation of nitrotyrosines (NO2-Tyr). Alternatively, MPO can use H2O2 to form hypochlorous acid (HOCl). Singlet oxygen (1O2) is formed upon the reaction of HOCl with H2O2.
Abbreviations: 1e−, one electron; 2e−, two electrons; BH4, tetrahydrobiopterin; Cl−, chloride anion; Fe2+, ferrous iron; H+, hydrogen cation; Tyr, tyrosine.