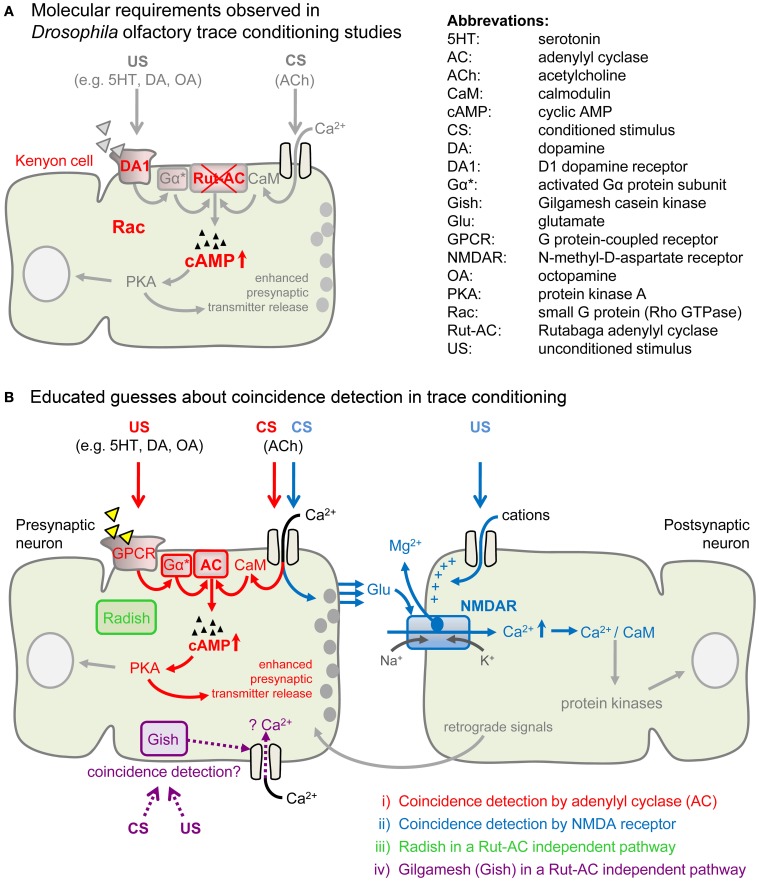
Figure 3.
Molecular requirements for trace conditioning and four non-exclusive models of possible coincidence detection. (A) Cellular and molecular requirements which were shown to contribute to Drosophila olfactory trace conditioning. Shuai et al. (2011) found that the targeted inhibition of Rac in the mushroom bodies increased trace-dependent memory formation. Also D1 dopamine receptor (DA1) expression in mushroom bodies was required for trace conditioning, as shown by rescue experiments (Shuai et al., 2011). Trace conditioning does not require the Rutabaga adenylyl cyclase (Rut-AC; Shuai et al., 2011), but delay and trace conditioning simulations both induced synergistic increases of cAMP (Tomchik and Davis, 2009). (B) Educated guesses about coincidence detection in delay conditioning might also apply to trace conditioning. (i) Presynaptic coincidence detection by an adenylyl cyclase (AC; shown in red). In the presynaptic neuron, the CS induces Ca2+ influx and Ca2+ binds to calmodulin (CaM). The US activates G protein-coupled monoaminergic receptors (GPCR) which activate the associated G protein (Gα). When Ca2+/CaM complex and activated G protein (Gα*) co-occur, the AC is activated more strongly than if they appear alone. This leads to an increased production of cAMP and to activation of protein kinase A (PKA) which enhances presynaptic transmitter release (Heisenberg, 2003). (ii) Postsynaptic coincidence detection by the N-methyl-D-aspartate-type glutamate (NMDA) receptor (shown in blue). The CS leads to presynaptic release of glutamate (Glu) which binds to the postsynaptic NMDA receptor. The US, on the other hand, induces the depolarization of the postsynaptic membrane, which allows for the removal of the Mg2+ block from the NMDA receptor channel. Opening of the NMDA receptor channel for Ca2+ influx is only possible when the CS and the US signal coincide. An elevation of the intracellular Ca2+ level leads to the activation of several kinases, inducing synaptic plasticity. The NMDA receptor is involved in delay conditioning in Drosophila (Miyashita et al., 2012) and was also shown to be involved in trace conditioning in vertebrates (Gilmartin and Helmstetter, 2010; Czerniawski et al., 2012). A possible role in insect trace conditioning has not yet been investigated. (iii) Radish (shown in green) is involved in a Rut-AC independent pathway (Isabel et al., 2004; Folkers et al., 2006) and might contribute to trace conditioning. (iv) Gilgamesh (Gish) (shown in purple), a casein kinase I γ homolog in flies, is required for short-term memory formation in Drosophila olfactory delay conditioning, functioning independently of Rut-AC and the cAMP pathway (Tan et al., 2010). Hypothetically, Gish mediates increased Ca2+ influx upon CS–US coincidence and thus might be a pathway for Rut-AC independent trace conditioning.