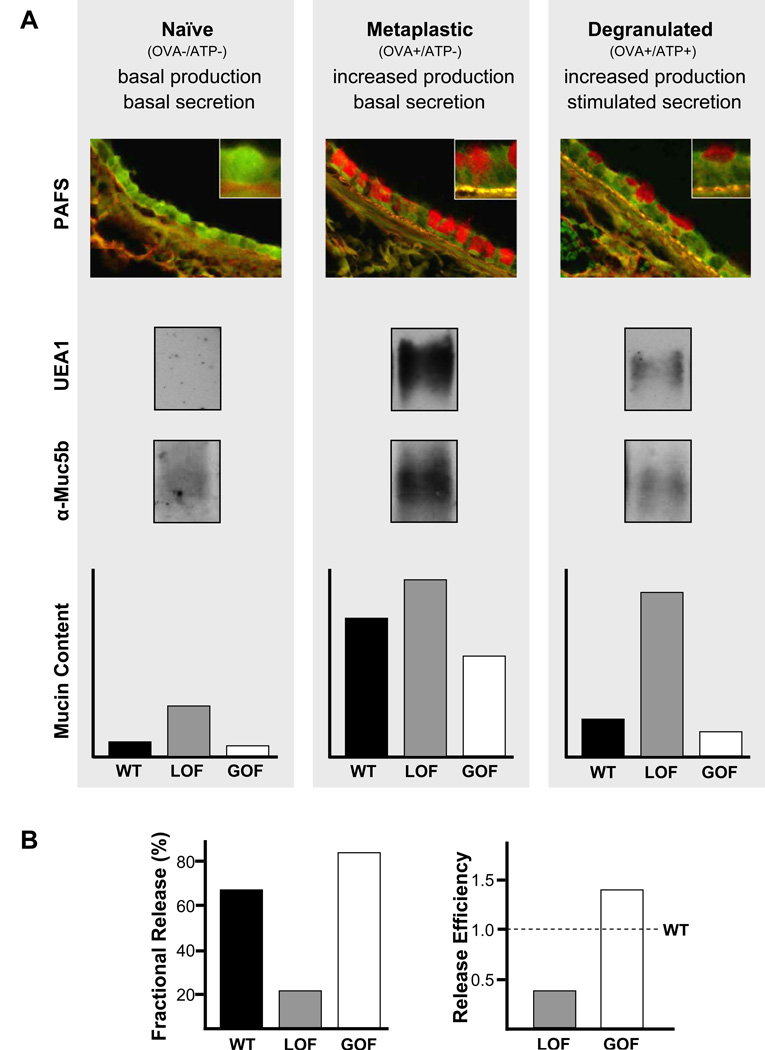
Figure 3.
Assessment of mucin secretion in vivo by measurement of intracellular mucin content. (A) Airway[DT11] epithelial mucin content is measured in three conditions – naïve, metaplastic, and [m12][MACC13]degranulated. These three conditions can be induced by treatment or not with ovalbumin immunization and airway challenge (OVA), followed or not by airway challenge with ATP. Treatment with OVA results in increased mucin production, and treatment with ATP induces acute mucin secretion. Intracellular mucin content after treatment can be measured by image analysis of tissue sections stained with PAFS, or lung tissue extracted with guanidinium hydrochloride, electrophoresed through 1% agarose, then transferred to nitrocellulose and probed with UEA1 lectin to detect Muc5ac or antibodies against Muc5b (all shown for wild-type mice only). Intracellular mucin content measured in each of these conditions (naïve, metaplastic, degranulated) by any of these methods is illustrated for wild-type (WT) mice (black bars), and for mice with a loss-of-secretory-function (LOF, grey bars) or gain-of-secretory-function (GOF, white bars) due to genetic mutation or pharmacologic treatment . (B) For further comparison of differences in stimulated secretory function between WT and LOF or GOF animals, “fractional release” may be calculated as mucin content in degranulated animals as a percentage of mucin content in metaplastic animals (left), or “release efficiency” may be calculated as fractional release by LOF and GOF animals divided by fractional release by WT animals (right).