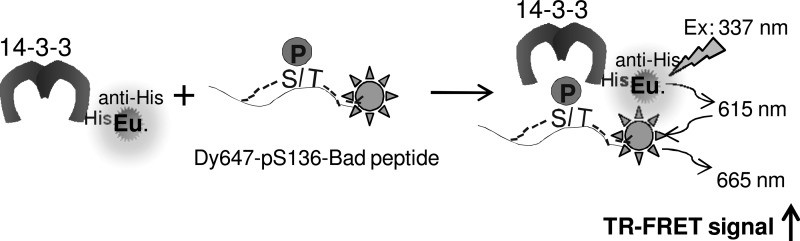
Fig. 1.
Design of a time-resolved fluorescence resonance energy transfer (TR-FRET) assay for monitoring the 14-3-3/pBad peptide interaction. His-14-3-3 protein is indirectly labeled with a long-lived fluorophore, Europium (Eu), through an anti–His-Eu antibody (PerkinElmer) to serve as a FRET donor. A phosphopeptide derived from Bad (pS136-Bad) is directly labeled with Dy647 to serve as a FRET acceptor. Interaction of 14-3-3 with pS136-Bad brings two fluorophores into proximity. On excitation at 337 nm, the energy emitted from the Eu donor is transferred to the Dy647 acceptor, leading to the generation of a FRET signal.