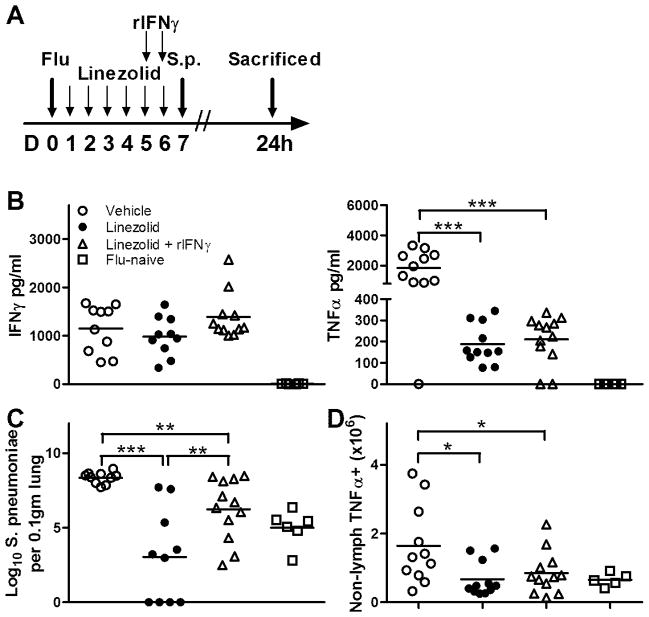
FIGURE 5.
Linezolid-induced effects on post-influenza secondary bacterial infections is IFNγ-dependent. (A) Animals were intranasally infected with influenza virus and dosed twice daily with linezolid (80mg/kg) or vehicle as depicted. At days 5 and 6 post-infection, a subset of mice that were receiving linezolid were given intranasal instillations of 2ng rIFNγ, with the remainder of the mice receiving vehicle only. At 18h after the last IFNγ dose, animals were intranasally challenged with S. pneumoniae A66.1 as described in the Methods section and sacrificed at 24h following bacterial challenge. An additional group of mice were challenged with S. pneumoniae without prior influenza infection. (B) Bronchoalveolar lavage fluid was collected and analyzed for expression of IFNγ (left panel) and TNFα (right panel) by ELISA. (C) To assess bacterial burden at 24h post-infection, lung digest samples were plated for CFU enumeration on blood agar plates and expressed per 0.1gm of lung. (D) To compare the number of non-lymphocytes that were producing TNFα, intracellular cytokine staining was performed on lung digest samples, and the number of cells within the non-lymphocyte gate that fluoresced positive for TNFα as analyzed by flow cytometry are depicted. Horizontal lines represent mean values, and each symbol is representative of one animal. Data was pooled from multiple experimental replicates, and means were compared using 1-way ANOVA along with the Student Newman-Keuls post hoc test. *p<0.05, **p<0.01, ***p<0.001