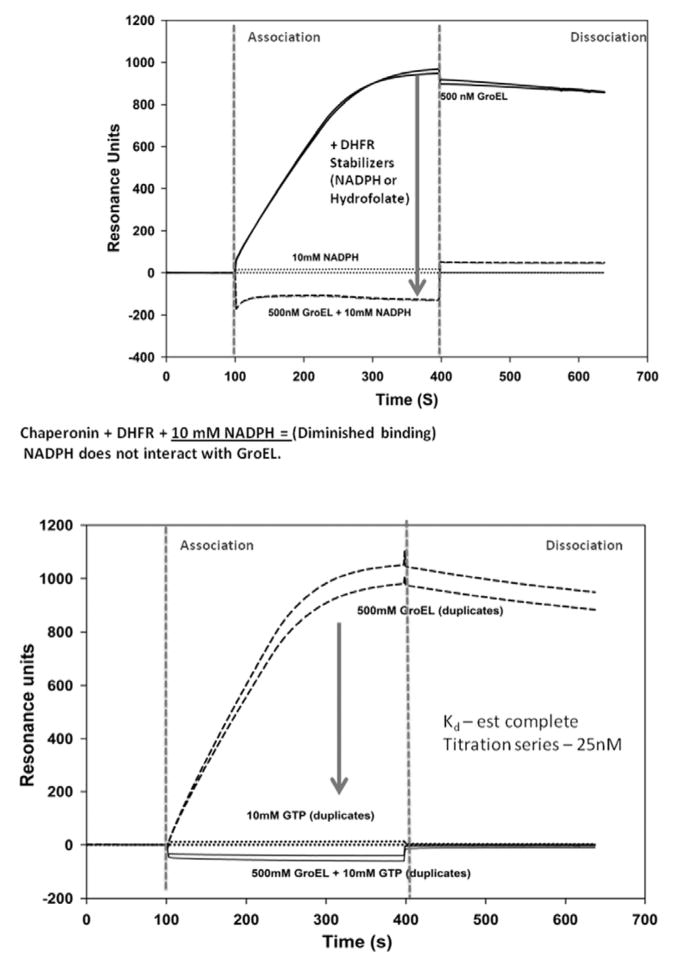
Fig. 6.
In Panel A, one can rapidly identify naturally occurring protein binding ligands that result in the stabilization of immobilized Dihydrofolate reductase (DHFR). As demonstrated by Viitanen et al., (1991), the presence of a stabilizing ligand such as NADPH results in a marked decline in DHFR binding to the GroEL chaperonin. This outcome results in a decrease in the SPR signal due to the decrease in GroEL chaperonin binding to the immobilized DHFR. In Panel B, the SPR signal from GroEL chaperonin binding to the destabilized, immobilized CFTR-NBD1 protein was significantly diminished when a known stabilizing ligand such as GTP was present in solution. Importantly, the GroEL chaperonin exclusively binds ATP and whereas GTP fails to bind to the chaperonin.