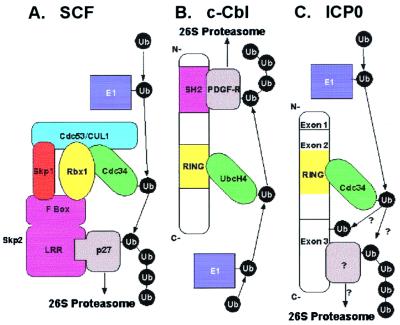
Figure 6.
Models for E3 ubiquitin ligase function. E1 ubiquitin-activating enzymes are shown in dark blue, E2 ubiquitin-conjugating enzymes in green, RING finger subunits and domains in yellow, substrate-binding subunits and domains in magenta, and substrates in gray. (A) Multicomponent E3 complex modeled after SCF (adapted from ref. 40). The substrate is recruited to the complex by a specific substrate-binding domain within a protein that contains an F-box motif. The F-box interacts with SCF components Skp1 and Rbx1. Recruitment of substrate p27 to SCF by the leucine-rich repeat (LRR) of the F-box protein Skp2 is depicted here. Other F-box proteins recruit different substrates (reviewed in ref. 41). SCF contains cullin 1 (CUL1), whereas other cullins are components of other multicomponent E3s. The cullin serves as a scaffold to bind Skp1, the RING finger protein Rbx1, and the E2 cdc34, which also interacts the RING finger. (B) RING finger unimolecular E3 ubiquitin ligase modeled after c-Cbl (adapted from ref. 25). The RING finger interacts with and allosterically activates the E2, UbcH4 (35). A Src-homology 2 (SH2) domain binds phosphotyrosine, such as platelet-derived growth factor receptor β (PDGF-Rβ). (C) Proposed model for ICP0 unimolecular E3 ubiquitin ligase activity. Cdc34 binds the RING finger domain in ICP0-exon 2. Unidentified substrates may bind exon 3, facilitating the transfer of ubiquitin from E2 to the substrate. Also, it is possible that ICP0 E3 activity catalyzes regulatory self-ubiquitination, which does not target ICP0 for degradation.