Figure 8.
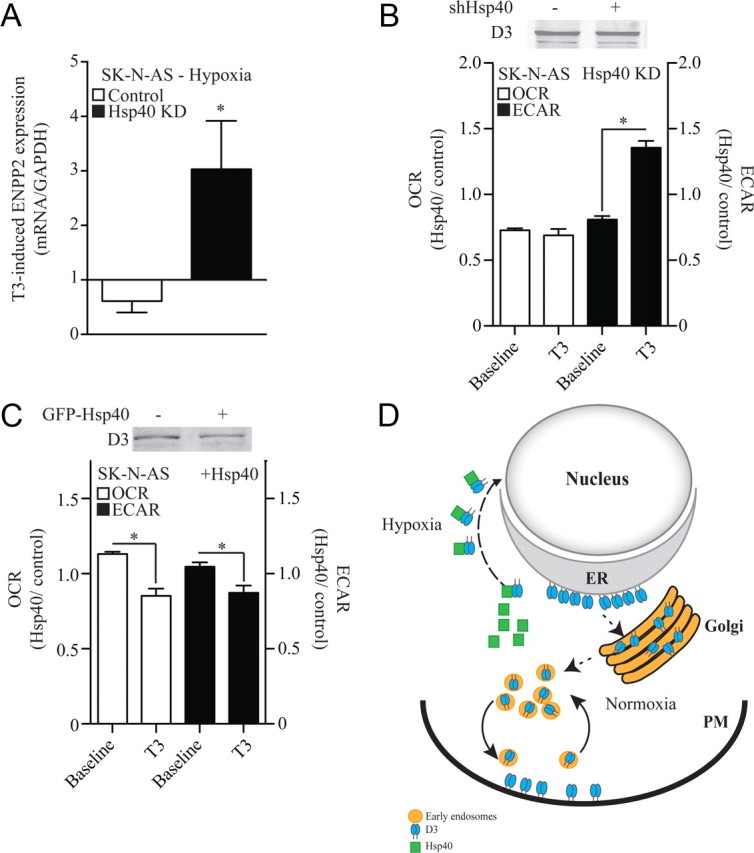
Nuclear D3 import dampens thyroid hormone signaling and slows down cellular metabolism. A, Effect of Hsp40 knockdown (KD) on the T3 inducibility of ENPP2 gene expression in hypoxic SK-N-AS cells; hypoxia and 50 nm T3 treatments were simultaneous and lasted for 24 h. Results are presented as a ratio between the T3 induction during hypoxia and the T3 induction during normoxia. B, OCR and ECAR (glycolysis) of SK-N-AS cells transiently expressing a control or an Hsp40 shRNA; 15,000 cells/well were plated and baseline values for both OCR and ECAR were measured as previously described (Simonides et al., 2008). Results are expressed as the OCR or ECAR ratio between Hsp40 KD and the control constructs at two different times: baseline and after treatment with 50 nm T3 for 24 h; data are presented as mean ± SEM; n = 30 per group; *p < 0.01 versus baseline reading by Student's t test. C, Same as in B except that studies were performed in cells transiently expressing a GFP-Hsp40. Both in B and C, D3 protein level did not change. D, Model explaining how O2 availability and Hsp40 regulate D3 subcellular localization. When O2 is available, ER-born D3 reaches the Golgi system and plasma membrane (PM), where it is internalized and recycled. During hypoxia, there is Hsp40-mediated nuclear import of D3, thus placing D3 physically closer to the thyroid hormone receptor.
