Figure 4.
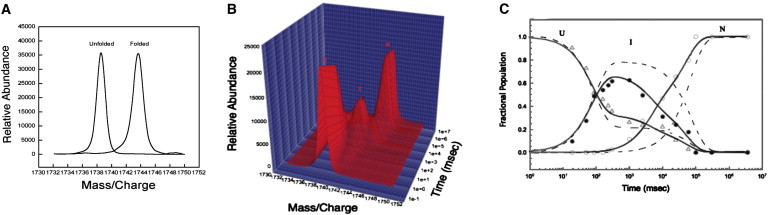
(A) Plot of the mass difference between the unfolded and folded F42W IL-1β mutant protein. The masses differ by 48 Da, which is due to the trapping of stable deuterium atoms in the folded state of the protein. The mass/charge ratio corresponds to the +10 charge peak. (B) Plot of the hydrogen-exchange data for the F42W mutant from 20 msec to 300 s after the initiation of folding. The decay of the unfolded population with the increase of an intermediate is evident along with the formation of the native state. The time is plotted in milliseconds on a logarithmic scale where the mass/charge ratio corresponds to the +10 charge peak. (C) Plot of the population versus time for the folding of F42W/W120F. The populations of U, I, and N are indicated by open triangles, solid circles, and open circles, respectively. The lines represent the fit of the data to a parallel pathway model where the intermediate folds to the native state by two discrete paths. The dashed line represents the various populations for WT IL-1β as represented in (23).
