Figure 4.
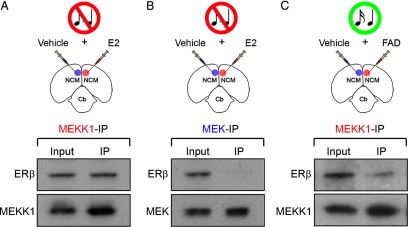
ERβ directly associates with MEKK1, but not MEK, as a result of locally derived E2 in NCM. A–C, The top panels depict camera lucida drawings of coronal sections illustrating the experimental design for co-immunoprecipitation studies. Animals were either unilaterally infused with E2 (A, B), or the aromatase inhibitor FAD (C) (n = 4). Control hemispheres for each group were infused with vehicle. NCM samples obtained from both control and experimental hemispheres, for each group, were immunoprecipitated with an anti-MEKK1 or an anti-MEK antibody. The antibodies used for the immunoprecipitation procedure are color coded. The lower panels show Western blots of both input (pre-immunoprecipitation) and immunoprecipitated samples probed with an ERβ antibody. Note that in E2-challenged hemispheres, ERβ is detectable in samples that were immunoprecipitated with the anti-MEKK1 antibody (A; top), but not with the anti-MEK antibody (B; top). These findings demonstrate that ERβ is associated MEKK1, but not MEK. In addition, suppression of the local production of E2 in NCM by infusion of the aromatase inhibitor FAD significantly decreases the levels of MEKK1 associated with ERβ in samples of animals stimulated with 5 min of song playbacks (C; top). Importantly, MEKK1 and MEK can be identified in the input, as well as recovered in immunoprecipitated samples (A–C; bottom). Of note, due to protein loss that typically occurs as a result of the immunoprecipitation procedure, Western blots shown in this figure carry ∼30× more protein in immunoprecipitated samples relative to inputs, as appropriate.
