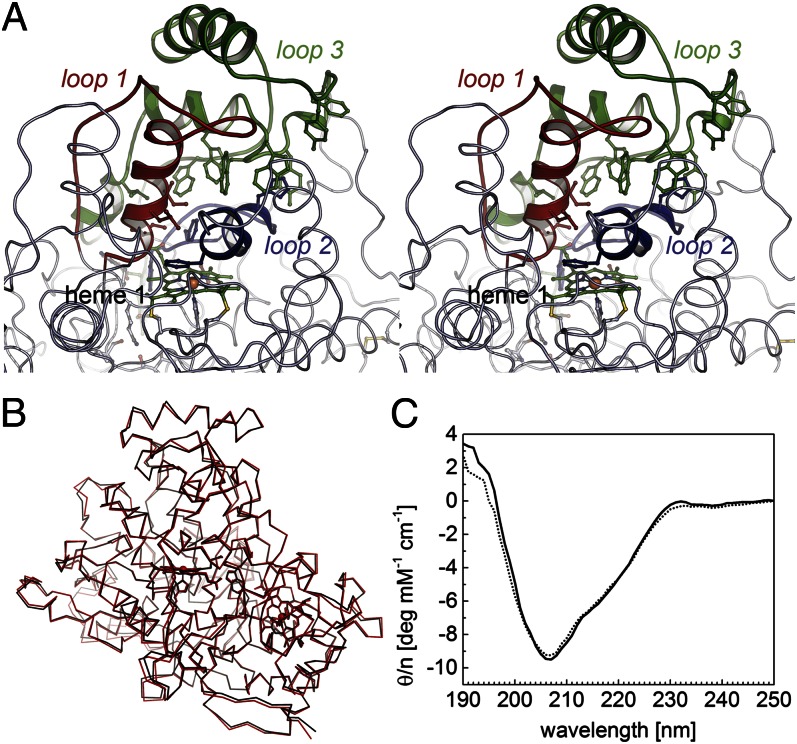
Fig. 5.
Architecture of the regulatory loop regions and possible active site access. (A) The topological equivalents of loop regions 1 (red), 2 (blue), and 3 (green) that rearrange on reductive activation of Ccp peroxidases are extended in RoxA and form a dome-like structure above the distal face of heme group 1. Note the chain of five tryptophans and one tyrosine residues in loop 3, as well as H312 and Y462, which can form H bonds with the terminus of the substrate chain and thus may act as the molecular ruler required to generate the trimeric product ODTD. (B) Superposition of an oxidized (black) and a dithionite-reduced (red) structure of RoxA with no major loop rearrangements. (C) CD spectra of oxidized (solid line) and dithionite-reduced (dotted line) RoxA showing that the conformation of the enzyme is independent of its redox state (see also Fig. S2).