Figure 3. ChlR1 is required for efficient DSB repair.
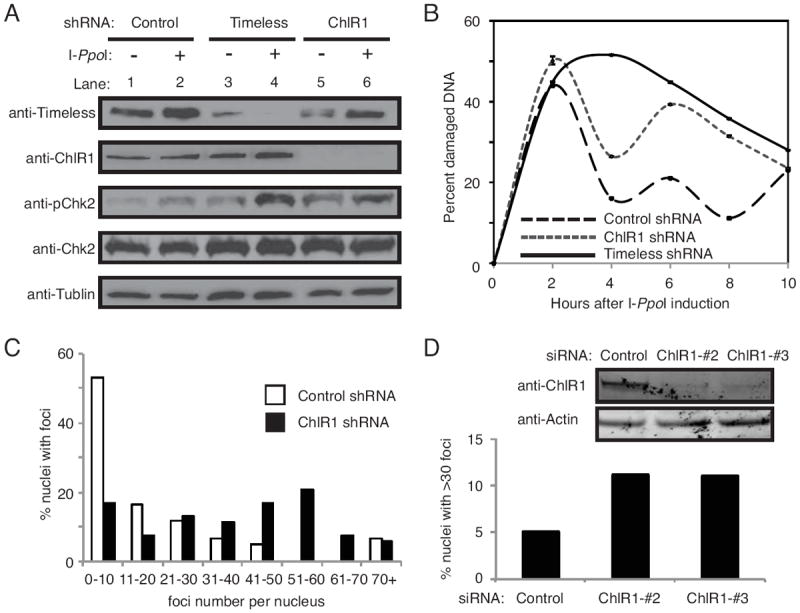
(A) Western blot analysis of cell extracts prepared from HeLa cells stably expressing the indicated shRNAs. Timeless or ChlR1 was efficiently downregulated. Cell extracts were collected before tamoxifen treatment (-), and 8 hours after tamoxifen treatment (+) to assess activation of DNA damage response by I-PpoI nuclear relocalization. Tamoxifen-dependent induction of I-PpoI induced phosphorylation of Chk2 (pChk2). (B) Cells were collected at the indicated times after I-PpoI induction, and genomic DNA was prepared. Triplicate real-time PCR reactions were run by amplifying the I-PpoI target site on chromosome 1, along with a site adjacent to the I-PpoI target site for normalization. ΔΔCT values were determined, and represented as the percentage of damaged DNA at the I-PpoI target. Error bars represent standard deviations. (C) HeLa cells stably expressing the indicated shRNA were treated with 20 μg/ml of bleomycin for 4 hours. Cells were washed, returned to fresh medium without bleomycin for 18 hours, and stained with the 53BP1 antibody. Quantification of 53BP1 foci in these cells were performed as described in Fig 2C. (D) HeLa cells were transiently transfected with the indicated siRNA oligonucleotides and treated with 3 μg/ml bleomycin for 1 h. Cells were then washed, returned to fresh medium for 7 hours, and stained with the 53BP1 antibody. Percent nuclei with more than thirty 53BP1 foci is shown. Representative results of repeat experiments are shown.
