Abstract
Background
Familial influences on remission from alcohol use disorder (AUD) have been studied using family history of AUD rather than family history of remission. The current study used a remission phenotype in a twin sample to examine the relative contributions of genetic and environmental influences to remission
Method
The sample comprised 6183 twins with an average age of 30 years from the Australian Twin Registry. Lifetime history of alcohol abuse and dependence symptoms and symptom recency were assessed with a structured telephone interview. AUD was defined broadly and narrowly as history of two or more or three or more abuse or dependence symptoms. Remission was defined as absence of symptoms at time of interview among individuals with lifetime AUD. Standard bivariate genetic analyses were conducted to derive estimates of genetic and environmental influences on AUD and remission
Results
Environmental influences alone accounted for remission in males and for 89% of influences on remission in females, with 11% due to genetic influences shared with AUD, which decreased the likelihood of remission. For women, more than 80% of influences on remission were distinct from influences on AUD, and environmental influences were from individual experiences only. For men, just over 50% of influences on remission were distinct from those on AUD, and the influence of environments shared with the co-twin were substantial. The results for the broad and narrow phenotypes were similar
Conclusions
The current study establishes young adult remission as a phenotype distinct from AUD and highlights the importance of environmental influences on remission
Keywords: Alcohol dependence, alcohol use disorder, remission, twins
Introduction
Genetic influences on alcohol use disorder (AUD) have been estimated to account for 40–60% of the variance in risk (Heath et al. 1997; Prescott & Kendler, 1999; Prescott et al. 1999; Knopik et al. 2004), but genetic influences on remission from AUD have been virtually ignored. Although family history of alcohol dependence has been included as a covariate predicting remission in population-based (Dawson, 1996; Dawson et al. 2005, 2007) and clinical samples (Bottlender & Soyka, 2005), family history of remission, as distinct from family history of AUD, has not been examined.
Family history of alcohol dependence seems to have little or no influence on remission status in high-risk and population-based samples. Knop et al. (2007) examined associations of paternal alcoholism with alcohol dependence and remission in the all-male Danish Longitudinal Study on Alcoholism, in which members were assessed periodically from birth to age 40 years. Subjects whose fathers had documented histories of alcohol dependence were compared to socio-demographically matched subjects whose fathers had no such histories. Remission was defined as at least 6 months of abstinence from alcohol, or some use but no symptoms of alcohol dependence. Seventy percent of men with a history of alcohol abuse or dependence were in remission at the 40-year follow-up. Paternal alcohol dependence was associated with higher rates of lifetime alcohol dependence but not with remission, contrary to the authors' expectation that sons of alcoholic fathers would have lower rates of remission due to their higher familial risk. These authors suggest that different sets of genes may influence the development versus remission of alcohol dependence, or that genetics may have a larger influence on the development of dependence whereas psychosocial influences may be more salient for remission (Knop et al. 2007). The same sample was used to examine associations of 361 putative predictors of alcohol dependence with remission status at the 40-year follow-up, with the aim of identifying pre-morbid endophenotypes for alcohol dependence (Penick et al. 2010). All but four of the measures tested were collected before the development of an AUD, during the perinatal, early school-age and late adolescent periods. Of the 361 measures, only 18 had univariate associations (p ≤ 0.10, uncorrected for multiple testing) with remission status at the 40-year follow-up; family history of alcohol problems was not among them (Penick et al. 2010). Studies with shorter follow-up periods have similar results. History of alcohol dependence in first-degree relatives had no association with remission in a group of American Indians remitted for at least 6 months (Gilder et al. 2008). In a clinical sample followed for 36 months after intensive out-patient treatment, family history of alcohol dependence did not predict relapse to alcohol (Bottlender & Soyka, 2005).
Several studies in a national probability sample have also found scant evidence that family history of AUD influences remission. Among individuals with lifetime alcohol dependence who participated in the first wave of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), family history of alcoholism had no association with abstinent recovery but was associated with slightly increased odds for non-abstinent recovery (Dawson et al. 2005). Data from the second wave of the NESARC were used to examine correlates of relapse among individuals who were in remission from prior-year alcohol abuse or dependence at their first interview and who were reinterviewed approximately 3 years later. Family history of alcoholism had no association with remission (Dawson et al. 2007). Another analysis using NESARC data found that family history of a substance use disorder, defined as any alcohol or drug use disorder in first-degree relatives, had no association with remission from lifetime alcohol dependence (Lopez-Quintero et al. 2011).
All of the above-referenced studies measured familial influence on remission as family history of AUD, not family history of remission. The unstated assumption underlying use of this measure, rather than a measure more closely matching the phenotype under study, is that familial alcoholism will similarly affect both the development and the remission of AUD. It is possible, however, that familial influences on remission may differ from those on AUD after accounting for family history of AUD.
The current study used a remission phenotype to examine the relative contributions of genetic and environmental factors to AUD and to remission in a population-based sample of young adult twins. It is the first study to our knowledge to examine the heritability of remission using a direct measure of remission rather than familial alcohol problems. Additionally, this study provides a direct test of genetic and environmental influences on remission that are shared with AUD.
Method
Participants
Participants were members of the young adult cohort of the Australian Twin Registry, a volunteer twin panel maintained by the Australian National Health and Medical Research Council (the older cohort of the registry, born from 1944 to 1963, provided data for an earlier analysis of the heritability of alcohol dependence; see Heath et al. 1997). All twins in the current study were born in Australia between 1964 and 1971 and were recruited into the Australian Twin Registry through mass media and school system appeals to their parents between 1980 and 1982 (Lynskey et al. 2003). Twins participated in a telephone interview during 1996–2000, when their mean age was 30 years (range 24–36 years). Data from 2711 pairs of twins and 761 singletons with complete data on alcohol abuse and dependence symptoms were used in this analysis (55% female).
Assessment
The Semi-Structured Assessment for the Genetics of Alcoholism (Bucholz et al. 1994; Hesselbrock et al. 1999) was adapted for telephone use in Australia and administered to participants by trained lay interviewers. Informed consent was obtained from all participants prior to the interview. The institutional review boards of Washington University School of Medicine, St Louis, MO, USA and the Queensland Institute of Medical Research, Brisbane, Australia approved the informed consent procedure.
Aud
DSM-IV-defined alcohol abuse and dependence symptoms, including ages at onset and recency of each symptom, were assessed for all individuals who had ever had a full drink of alcohol. A five-level ordinal variable was constructed based on number of abuse and dependence symptoms endorsed (0, 1, 2, 3 or 4 or more); this variable was used in the variance components analysis described below (symptom categories were combined for descriptive purposes in tables). Lifetime AUD was operationalized in accordance with the proposed DSM-5 definition of AUD as occurrence of at least two of the 11 AUD symptoms (APA, 2010); however, the clustering of symptoms within a 12-month period was not imposed. This decision was based on findings from a previous analysis of these data that found a genetic correlation of 0.99 for alcohol dependence (AD) symptom count with a separate measure of symptom clustering, indicating that clustering contributed almost no additional genetic information (Grant et al. 2009). That study also found a genetic correlation of 0.96 for AD symptom count and alcohol abuse, suggesting that abuse and dependence symptoms tapped the same underlying genetic liability. In the current study, therefore, the use of AUD symptom count without clustering is supported by evidence showing that abuse and dependence are genetically correlated and that symptom clustering adds little genetic information to the phenotype. The AUD phenotype is also consistent with evidence from other twin samples that the magnitude of genetic influences on risk are similar for narrowly defined alcohol dependence and for broadly defined problem drinking (Prescott & Kendler, 1999; Prescott et al. 1999).
Remission
Remission was operationalized as absence of symptoms at the time of interview and was conditional on the presence of lifetime AUD as defined above. A narrower phenotype based on lifetime presence of three or more AUD symptoms was also created and analyses were repeated using this stricter definition. Remission was defined so that symptom recency was at least 1 year less than current age, consistent with previous studies requiring a minimum of 6 months remission (Knop et al. 2007; Gilder et al. 2008; Penick et al. 2010). Early remission was defined as ≤12 months with no symptoms (i.e. age at most recent symptom was 1 year less than current age), and sustained remission as >12 months with no symptoms (i.e. age at most recent symptom was at least 2 years less than current age). A three-level variable representing remission status (none, early, sustained) was created to test whether early and sustained remission were statistically distinct categories. This variable was regressed on two dummy variables representing co-twin status on early and sustained remission in a multinomial logistic regression (with no remission as the reference category) and planned post-hoc tests were used to test whether co-twin early and sustained remission were differentially associated with twin early and sustained remission. Co-twin early and sustained remission were not differentially associated with twin early or sustained remission from two or more [Wald χ2(3)=0.67, p=0.88] or from three or more AUD symptoms [Wald χ2(3)=0.39, p=0.94]. Remission was coded as binary thereafter, with 1 representing individuals with any remission (early or sustained). Individuals who did not endorse two or more AUD symptoms (for broadly defined remission, n=3343) or three or more (n=4380) were coded as missing on remission, consistent with the two-stage model used to decompose variance into genetic and environmental components, described below.
Statistical Analysis
Twin modeling, based on biometrical genetics (Neale & Cardon, 1992), uses the natural contrast between monozygotic (MZ) twins (who share 100% of their genetic material) and dizygotic (DZ) twins (who share on average 50% of their segregating genes) to estimate genetic and environmental influences on behavior. In the current study, proportions of variance in risk for AUD and remission accounted for by additive genetic influences (A), environmental influences shared by twins, such as early family environment (C), and unique environmental influences, which distinguish twins from one another (E), were estimated using a two-stage model in which sources of variance on remission are partitioned into those shared with AUD and those specific to remission. All participants had data for the five-level AUD symptom variable, which represented the first stage of the model. Remission represented stage 2 of the model, with individuals having 0–1 AUD symptoms, or 0–2 in the case of more narrowly defined AUD, set to missing because, by definition, only those with AUD can remit from them. Under this two-stage model, twin pairs discordant for alcohol problems still contribute information about genetic influences on AUD and about shared genetic and environmental variance between AUD and remission (Heath et al. 2002). A test of bivariate normality confirmed that a single dimension of liability underlay the AUD variable, so that a model using full information maximum likelihood estimation could be used. A bivariate Cholesky model was then fit to the data using the MX statistical package (Neale et al. 2003). The Cholesky model estimates sources of variance unique to AUD and remission in addition to variance common to the phenotypes. Information from opposite-sex twin pairs was estimated by allowing separate thresholds for males and females within opposite-sex pairs. First, a saturated model was fitted that estimated parameters separately for males and females; subsequent models were compared to this model using change in log likelihood relative to change in degrees of freedom and Akaike's Information Criterion (AIC). A model that constrained male and female parameters to be equal did not provide a good fit to the data and so parameters were estimated separately by gender in subsequent models. Next, models were estimated that tested the following hypotheses regarding sources of variance in risk for AUD and remission: (i) no environmental factors shared by twins influence risk for AUD or remission (AE model for AUD and remission), (ii) influences on remission are distinct from influences on AUD (i.e. the phenotypes share no genetic or environmental variance), (iii) there are no genetic influences on remission (CE model for remission), and (iv) there are no genetic or shared environmental influences on remission (E model for remission). The fit statistics from these models were used to identify the best-fitting models for males and females in the final model. All models were adjusted for age using a binary variable representing individuals aged ≥30 years because these individuals were more likely to be remitted from two or more [<30 years: 35.5%; ≥30 years: 44.0%; odds ratio (OR) 1.4, 95% confidence interval (CI) 1.2–1.7] or three or more AUDsymptoms (<30years:27.2%;≥30 years: 38.8%; OR 1.7, 95% CI 1.4–2.1).
Results
Individuals with 0–1, 2 and ≥3 lifetime symptoms were similar in age but their life situations, as reflected in their educational, work and marital status at the time of interview, were very different (Table 1). Individuals with two or more lifetime AUD symptoms had less education, were less likely to have children, and were more likely to be separated or divorced, or to live as though married, than were individuals with just 0–1 symptoms. In addition, these individuals reported an earlier onset of alcohol problems, as reflected in younger ages at regular drinking and onset of first AUD symptom, and a larger maximum number of drinks consumed in a 24-h period (Table 1). The co-twins of twins with two or more symptoms were more likely to report them as having alcohol problems than were co-twins of twins with 0–1 symptoms. Individuals with three or more symptoms were more likely to report alcohol problems in one or both parents.
Table 1. Demographic and alcohol use characteristics of a population-based sample of young adult twins, by number of lifetime AUD symptoms.
| 0–1 AUD symptom (n=3343) | 2 AUD symptoms (n=1036) | ⩾3 AUD symptoms (n=1804) | |
|---|---|---|---|
| Female | 65.5A | 52.7B | 37.8C |
| Age ⩾30 years | 58.2 | 57.6 | 58.2 |
| Education | |||
| Less than high school | 18.8A | 22.3A | 28.6B |
| High school only (ref.) | 42.4 | 43.9 | 44.7 |
| Some college or higher | 38.8A | 33.7B | 26.7C |
| Work status | |||
| Student/unemployed | 4.4A | 5.0A | 8.9B |
| Part/full-time (ref.) | 79.6 | 82.6 | 83.1 |
| Homemaker | 16.0A | 12.4B | 8.0C |
| Marital status | |||
| Married (ref.) | 56.8 | 49.6 | 38.6 |
| Separated/divorced | 6.2A | 7.2B | 7.6C |
| Never married | 37.0A | 43.2B | 53.8C |
| Age at marriage | 24.8 (3.2)A | 25.6 (3.1)B | 26.0 (3.2)C |
| Living as though married | |||
| Currently | 11.5A | 15.6B | 19.3C |
| Formerly | 7.5A | 10.9B | 17.6C |
| Any children | 46.3A | 42.1B | 38.4B |
| Age regular drinking (years) | 18.8 (2.8)A | 18.0 (2.2)B | 17.3 (2.0)C |
| Maximum drinks in 24 h | 12.1 (9.0)A | 19.1 (12.1)B | 27.0 (15.3)C |
| Onset of first AUD symptom | 20.1 (3.4)A | 18.8 (3.1)B | 17.4 (2.6)C |
| Co-twin report of alcohol problemsa | 7.4A | 19.3B | 44.6C |
| Alcohol problems in parent(s) | |||
| One parent only | 24.5A | 25.9A | 35.9B |
| Both parents | 2.3A | 3.1A | 6.1B |
AUD, Alcohol use disorder; ref., reference.
Different capital superscripts across symptom categories indicate statistically significant differences between groups at p<0.05, based on Wald tests following multinomial regressions of AUD symptom categories on individual variables, adjusted for familial clustering.
Values given as percentage or mean (standard deviation).
For complete twin pairs.
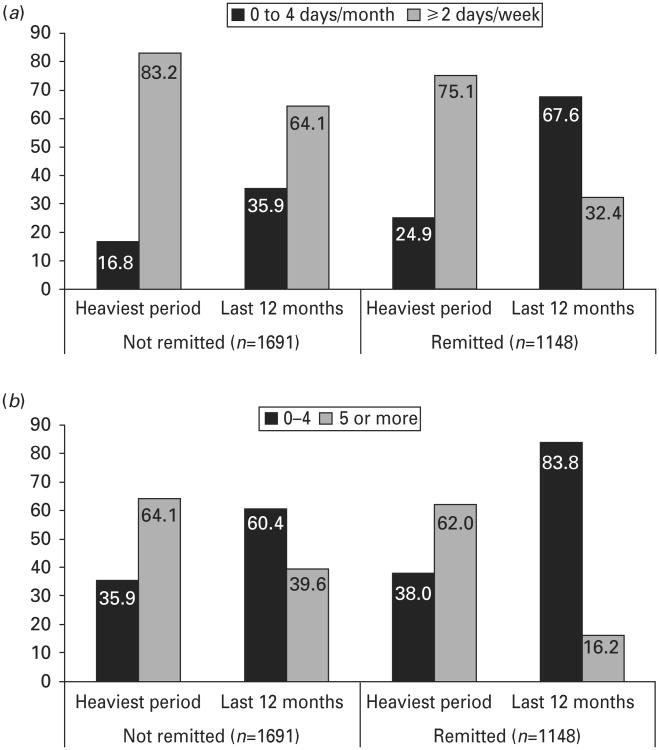
Treatment for alcohol problems was uncommon among individuals with two or more (2.5%) or with three or more AUD symptoms (3.8%). Among individuals with two or more symptoms, 32.9% of men and 50.3% of women were in remission (gender difference significant at p<0.001); for men and women with three or more symptoms, rates of remission were 28.3% and 43.3% respectively (p<0.001). Individuals who were remitted from AUD had lower rates of lifetime DSM-IV alcohol dependence, a shorter duration of AUD, and more periods of abstinence than individuals with current symptoms (Table 2). The change in drinking pattern from the 12-month period of heaviest drinking to current drinking was greater for remitted than non-remitted individuals (Fig. 1). The proportion of individuals drinking two or more days a week, for example, decreased to a greater extent among remitted (from 75.1% to 32.4%) than non-remitted individuals (from 83.2% to 64.1%). Similarly, the proportions drinking five or more drinks per occasion dropped more for remitted than non-remitted individuals. Only 6.9% of remitted individuals reported abstinence in the past year (four non-abstinent individuals also reported abstinence, probably because of the strict age requirement for remission of at least 1 year prior to current age).
Table 2. AUD symptom count, drinking frequency and quantity among individuals with ⩾2 AUD and ⩾3 AUD symptoms, by remission status.
| ⩾2 AUD symptoms | ⩾3 AUD symptoms | |||
|---|---|---|---|---|
|
|
|
|||
| Not remitted (n=1691) | Remitted (n=1148) | Not remitted (n=1191) | Remitted (n=612) | |
| Lifetime DSM-IV alcohol dependence | 52.7 | 35.1 | 74.8 | 65.8 |
| Duration of AUD (years)a | 8.8 (3.9) | 5.8 (3.7) | 9.5 (3.8) | 6.7 (3.6) |
| Number of times abstinent ⩾6 months | ||||
| Never | 72.6 | 52.5 | 73.1 | 50.5 |
| Once | 10.6 | 15.2 | 10.9 | 15.4 |
| Twice | 6.0 | 10.6 | 5.4 | 11.6 |
| Three times | 4.5 | 8.7 | 4.4 | 9.1 |
| Four or more times | 6.3 | 13.0 | 6.2 | 13.4 |
AUD, Alcohol use disorder.
Values given as percentage or mean (standard deviation).
Duration of AUD=time from onset of second AUD symptom to symptom recency.
Fig. 1.
(a) Frequency of drinking and (b) usual number of drinks per drinking occasion during the heaviest 12-month period of drinking and past 12 months, for individuals with two or more alcohol use disorder (AUD) symptoms, by remission status.
In describing the remaining results, we focus on AUD and remission phenotypes based on two or more AUD symptoms. The results for the narrower three-symptom phenotype are similar, and are displayed in the tables.
Twin pair correlations for AUD and remission (in twin pairs concordant for lifetime AUD), and ORs reflecting risk in one twin given the presence of AUD or remission in the other, are displayed in Table 3. The MZ correlation for AUD was more than twice the DZ correlation in female pairs but less than twice the DZ correlation in male pairs, raising the possibility of shared environmental in addition to genetic influences on AUD in males. In MZ female (MZF) pairs, twins of co-twins with an AUD had 3.8 times the risk of having an AUD themselves, relative to twins whose co-twins did not have an AUD; in DZF pairs, this increased risk was 1.6. The CIs for MZF and DZF twins did not overlap, suggesting a distinct difference by zygosity. This differentiation was not discernible in male (M) twins, where ORs for MZM and DZM pairs were more similar. Remission appeared to have familial influences in male pairs, with significant ORs in MZM twins showing increased chance of remission in twins of remitted co-twins, with a similar trend in DZM twins. By contrast, female and opposite-sex (OS) pairs showed little familial resemblance on remission. Significant negative correlations of co-twin remission and twin AUD in MZ twins indicated a decreased chance of remission among twins of co-twins with lifetime AUD.
Table 3. Tetrachoric correlations and odds ratios (ORs) showing twin pair associations for AUD and remission, by zygosity.
| Twin 1 phenotype/Twin 2 phenotype | ⩾2 AUD symptomsa | ⩾3 AUD symptomsb | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| n | r (S.E.) | OR (95% CI) | n | r (S.E.) | OR (95% CI) | |
| AUD/AUD | ||||||
| MZF | 690 | 0.47 (0.05) | 3.82 (2.73–5.35) | 690 | 0.57 (0.06) | 6.23 (4.04–9.60) |
| DZF | 503 | 0.19 (0.07) | 1.64 (1.12–2.38) | 503 | 0.36 (0.08) | 2.97 (1.82–4.87) |
| MZM | 484 | 0.49 (0.06) | 3.83 (2.62–5.60) | 484 | 0.58 (0.05) | 5.44 (3.63–8.16) |
| DZM | 389 | 0.44 (0.07) | 3.37 (2.21–5.15) | 389 | 0.42 (0.07) | 3.11 (2.04–4.74) |
| DZOS | 645 | 0.11 (0.06) | 1.35 (0.97–1.88) | 645 | 0.26 (0.07) | 2.12 (1.44–3.13) |
| Remission/Remission | ||||||
| MZF | 124 | 0.09 (0.14) | 1.26 (0.61–2.59) | 58 | 0.17 (0.21) | 1.57 (0.53–4.63) |
| DZF | 80 | 0.08 (0.17) | 1.23 (0.51–2.99) | 35 | −0.18 (0.27) | 0.62 (0.15–2.57) |
| MZM | 190 | 0.38 (0.11) | 2.92 (1.49–5.71) | 108 | 0.34 (0.16) | 2.67 (1.01–7.08) |
| DZM | 161 | 0.24 (0.13) | 1.91 (0.96–3.79) | 96 | 0.24 (0.17) | 1.93 (0.77–4.86) |
| DZOS | 152 | −0.08 (0.13) | 0.81 (0.40–1.64) | 74 | 0.19 (0.19) | 1.69 (0.57–5.02) |
| AUD/Remission | ||||||
| MZF | 236 | −0.29 (0.10) | 0.47 (0.28–0.79) | 124 | 0.03 (0.14) | 1.09 (0.53–2.24) |
| DZF | 181 | −0.12 (0.12) | 0.72 (0.40–1.30) | 94 | −0.11 (0.16) | 0.75 (0.32–1.77) |
| MZM | 274 | −0.22 (0.10) | 0.56 (0.32–0.95) | 183 | −0.29 (0.12) | 0.45 (0.23–0.88) |
| DZM | 228 | −0.17 (0.11) | 0.62 (0.35–1.12) | 157 | 0.08 (0.13) | 1.23 (0.61–2.5) |
| DZOS | 388 | −0.06 (0.08) | 0.85 (0.54–1.31) | 269 | −0.05 (0.11) | 0.86 (0.46–1.60) |
AUD, Alcohol use disorder; S.E., standard error; CI, confidence interval; MZF, monozygotic female; DZF, dizygotic female; MZM, monozygotic male; DZM, dizygotic male; DZOS, dizygotic opposite sex.
AUD defined as lifetime presence of two or more symptoms of abuse or dependence.
AUD defined as lifetime presence of three or more symptoms of abuse or dependence.
Shown in Table 4 are the standardized path coefficients for A, C and E influences on AUD and on remission (contingent on history of AUD) for saturated and best-fitting models (see Supplementary Tables S1 and S2 for fit statistics). Familial influences on remission were small to moderate, with all familial effects in males attributable to shared environment (C; accounting for 37% of the total variance), and all familial effects in women attributable to genetic factors (A; accounting for 11% of the total variance). The negative path coefficients for influences common to AUD and remission suggest that environmental (in males and females) and genetic influences (in females) associated with AUD inhibit the likelihood of remission.
Table 4. Standardized path coefficients with 95% confidence intervals for AUD, remission from AUD, and remission variance shared with AUD, showing two models: (i) remission from ⩾2 AUD symptoms, (ii) remission from ⩾3 AUD symptoms.
| ⩾2 AUD symptoms | ⩾3 AUD symptoms | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| A | C | E | A | C | E | |
| Saturated model | ||||||
| Male | ||||||
| AUD | 0.57 (0.31–0.71) | 0.45 (0.20–0.62) | 0.69 (0.64–0.74) | 0.57 (0.33–0.71) | 0.45 (0.19–0.62) | 0.69 (0.64–0.74) |
| Remission | 0.45 (0.00–0.70) | 0.31 (0.00–0.62) | 0.69 (0.56–0.83) | 0.00 (0.00–0.69) | 0.47 (0.00–0.64) | 0.73 (0.54–0.87) |
| AUD/Remission | −0.20 (–0.48 to 0.17) | −0.32 (–0.66 to 0.03) | −0.27 (–0.40 to –0.14) | −0.39 (–0.72 to 0.01) | −0.20 (–0.59 to 0.34) | −0.24 (–0.42 to –0.04) |
| Female | ||||||
| AUD | 0.70 (0.64–0.74) | 0.00 (0.00–0.20) | 0.71 (0.67–0.76) | 0.70 (0.65–0.74) | 0.00 (0.00–0.19) | 0.71 (0.67–0.76) |
| Remission | 0.00 (0.00–0.46) | 0.00 (0.00–0.43) | 0.86 (0.74–0.94) | 0.00 (0.00–0.59) | 0.14 (0.00–0.50) | 0.89 (0.69–0.99) |
| AUD/Remission | −0.35 (–0.51 to –0.20) | 0.28 (–0.03 to 0.47) | −0.25 (–0.40 to –0.09) | −0.31 (–0.53 to –0.06) | 0.23 (–0.18 to 0.52) | −0.20 (–0.44 to 0.08) |
| Final model | ||||||
| Male | ||||||
| AUD | 0.61 (0.49–0.69) | 0.39 (0.26–0.52) | 0.69 (0.64–0.74) | 0.57 (0.41–0.68) | 0.44 (0.28–0.58) | 0.69 (0.64–0.74) |
| Remission | N.E. | N.E. | 0.74 (0.63–0.84) | N.E. | N.E. | 0.76 (0.60–0.89) |
| AUD/Remission | N.E. | −0.61 (–0.71 to –0.45) | −0.29 (–0.42 to –0.16) | N.E. | −0.59 (–0.73 to –0.38) | −0.27 (–0.44 to –0.07) |
| Female | ||||||
| AUD | 0.70 (0.65–0.74) | N.E. | 0.71 (0.67–0.76) | 0.70 (0.65–0.74) | N.E. | 0.71 (0.67–0.76) |
| Remission | N.E. | N.E. | 0.91 (0.85–0.95) | N.E. | N.E. | 0.93 (0.82–0.99) |
| AUD/Remission | −0.33 (–0.47 to –0.17) | N.E. | −0.26 (–0.42 to –0.10) | −0.29 (–0.50 to –0.03) | N.E. | −0.22 (–0.45 to 0.06)a |
AUD/Remission, influences on remission shared with alcohol use disorder; N.E., not estimated (parameter was dropped).
This parameter could be dropped from the final model without a significant deterioration in fit [χ2(1)= 2.4, p=0.12]; however, Akaike's Information Criterion (AIC) indicated that the model shown provided the best fit to the data when compared with the saturated model (AIC for model shown=−8.24, after dropping additional parameter=−7.83). The loss of statistical significance thus probably results from reduced power due to use of more narrowly defined AUD.
Given the lack of genetic influences on remission in men and the minimal influence in women, associations of several measured environmental variables with remission were examined individually in logistic regression equations, followed by a multiple logistic regression and controlling for gender and lifetime AUD symptom count. The variables examined individually were lifetime treatment for alcohol problems (yes/no, p=0.25), marital status (married versus never married/separated/divorced, p<0.01), lifetime pregnancy status (yes/no, p<0.01), number of biological children [one only (p<0.01) versus two or more (p<0.01) versus none], religion (any affiliation versus none, p=0.73), education (greater than high school versus high school or less, p=0.34), work status [full-time (p<0.01) versus homemaker (p<0.01) versus unemployed], trauma history [childhood physical/sexual abuse (p<0.05) versus severe physical assault (p<0.01) versus witnessing injury/killing (p<0.05) versus no trauma], not having a close relationship with parents when aged 6 to 13 (versus having a close relationship, p=0.38), lots of tension between parents when aged 6 to 13 (versus some or no tension, p=0.06), and parental alcohol problems (any versus none, p=0.27). Variables associated with remission in the multiple logistic regression were alcohol treatment (OR 1.9, 95% CI 1.0–3.3), being married (OR 1.5, 95% CI 1.3–1.8), having one child (OR 1.7, 95% CI 1.3–2.1), and childhood physical or sexual abuse. There was a significant interaction of gender with childhood abuse indicating that women with histories of abuse were less likely than similar men to be in remission (OR 0.5, 95% CI 0.3–0.8).
To examine whether these measures might account for any of the environmental variance associated with remission, twin status on each measure was regressed on co-twin status on the same measure, twin remission status, and their interaction in same-sex twin pairs, adjusting for gender. A significant interaction would indicate that twin pair similarity on the environmental measure varied as a function of twin remission status. None of the interaction terms were significant. Finally, adjustment of the final genetic model for marriage and for childhood abuse did not substantially change the estimates of genetic and environmental influences on remission, suggesting that these variables did not account for much of the environmental variance associated with remission.
Discussion
To our knowledge, this is the first published study to examine the heritability of remission from AUD in a twin sample. Unlike previous studies that used familial AUD as a proxy for potential genetic influences on remission, the current study used a remission phenotype to test genetic influences on remission and also tested genetic and environmental influences shared with AUD. Environmental influences alone accounted for remission in men, with environments shared with the co-twin accounting for 37% of the variance. In women, environments unique to the individual accounted for 89% of influences on remission, with the remainder attributable to genetic effects associated with both AUD and remission. Variance common to AUD and remission had a negative influence on remission in men and women.
The dominant role of environmental influences in remission is consistent with the literature finding no association of familial history of AUD with remission (Dawson et al. 2007; Knop et al. 2007; Gilder et al. 2008) and stands in striking contrast to strong and coherent evidence for genetic influences on AUD (Kendler et al. 1994; Prescott et al. 1994, 1999; Heath et al. 1997; Prescott & Kendler, 1999; Knopik et al. 2004; Sartor et al. 2009, 2011). Genetic findings for AUD in the current study were broadly consistent with results reported previously for a narrower alcohol dependence phenotype for this sample (Knopik et al. 2004) and with a broadly defined alcohol use phenotype in a sample of US twins (Prescott & Kendler, 1999; Prescott et al. 1999). Shared environmental influences on AUD in male twins were detected in the bivariate analyses in the current study and before age 23 in a study of twins followed longitudinally (van Beek et al. 2012), but were not noted previously in reports of univariate genetic analysis (Heath et al. 1997; Prescott et al. 1999; Knopik et al. 2004). This is probably the consequence of the confounding of genetic non-additivity and shared environmental effects in the twin design, with the former decreasing and the latter increasing the DZ correlation relative to the MZ correlation, creating the potential for non-additivity to mask shared environmental effects and vice versa. In a multivariate analysis, where a second trait shows strong shared environmental influences (as proved to be the case for remission in males), it would not be unexpected to uncover shared environmental effects in the primary traits that were not detectable in a univariate analysis. Shared environmental influences on AUD were found in a twin study that used data from twins who had been hospitalized for alcoholism, defined as abuse, dependence or alcoholic psychosis (Prescott et al. 2007). In that study, twin resemblance for hospitalization may have effectively been a second trait that allowed the discernment of shared environmental influences, even in a univariate analysis.
Environmental influences on AUD had a negative association with remission, consistent with previous evidence that the development of AUD and remission from it represent distinct processes. Penick et al. (2010) found that only 18 of 300 variables measured before the development of alcohol abuse or dependence in males (Knop et al. 2003) were associated with both alcohol dependence and the failure to remit. Measures associated with the development of AUD (Knop et al. 2003) that had no association with the failure to remit from alcohol dependence at the 40-year follow-up (Penick et al. 2010) included socio-economic status of family at time of birth, familial alcohol problems, parental psychiatric problems, and number of life crises at age 19–20. The variables that were associated with failure to remit reduced to two factors: behavioral dyscontrol (with higher levels predicting failure to remit) and cognitive efficiency (with lower levels predicting failure to remit). In the current study, marriage was associated with remission from AUD and might reflect a life circumstance more closely aligned with behavioral control than dyscontrol. Marriage and stable relationships are associated with untreated remission from AUD in population-based samples and with better treatment outcomes in clinical samples (Bischof et al. 2001; Dawson et al. 2005; Moos & Moos, 2006). These associations may reflect life transitions that catalyze natural remission (Dawson et al. 2005, 2006; Moos & Moos, 2006), or they may be consequences of reduced drinking and life changes following remission.
Lifetime DSM-IV-defined alcohol dependence was less prevalent among remitted individuals, who also had a shorter duration of AUD, similar to evidence that individuals with less severe drinking histories are more likely to achieve remission (Moos & Moos, 2006; Penick et al. 2010). The remission rate of over 40% in this young sample is consistent with rates of remission in population-based (Lopez-Quintero et al. 2011), clinical (Charney et al. 2010) and high-risk samples (Schuckit et al. 2001; Ehlers et al. 2004). Among Mission Indians with a history of alcohol dependence, 61% were in remission, defined as no current symptoms, when interviewed for a cross-sectional study (Ehlers et al. 2004). In a high-risk family study of probands with alcohol dependence and their family members, 32% of individuals with alcohol dependence and 45% of those who met abuse criteria at baseline met no criteria 5 years later (Schuckit et al. 2001). A clinical study of 175 patients in treatment for alcohol abuse or dependence found that 43% of participants were abstinent 4 weeks after beginning out-patient treatment for AUD (Charney et al. 2010). In a population-based sample using two waves of data from the NESARC, 37% of 4781 individuals with alcohol dependence were remitted 10 years after its onset, and the cumulative probability of remission over the lifetime was 91% (Lopez-Quintero et al. 2011). The remission rate of 40% in the current sample is therefore a reasonable estimate consistent with findings in a variety of samples.
Evidence that the relative contributions of genetic and environmental influences to alcohol use shift over time may help to guide future studies of remission. Twin studies suggest that, during adolescence, environmental influences on alcohol initiation and use predominate (Han et al. 1999; Rose et al. 2001b), but that genetic influences increase with age (Viken et al. 1999; Rose et al. 2001a). A recent study in a longitudinal twin sample found that genetic and unique environmental influences on alcohol abuse and dependence symptoms increased from age 15–17 to age 30–32, while shared environmental influences decreased (van Beek et al. 2012). Perhaps remission follows a similar pattern, wherein early remission is influenced primarily by the environment, but in longer, sustained remission, genetic influences gain importance. This possibility has not yet been explored.
This study must be interpreted with caution for several reasons. The study relied on retrospective recall of age at symptom recency, upon which the remission variable was based. It is possible that an ordinal, rather than a binary, remission phenotype, such as years of abstinence, could provide more power to detect genetic influences on remission, but low rates of abstinence in this young adult sample prohibit its use as a representative phenotype for remission. The sample is relatively young and not yet through the period of risk for developing an AUD or remitting from one, and the possibility of subsequent relapse among remitted individuals in later adulthood cannot be excluded. However, the study does suggest that the environment is the predominant influence on remission in individuals early in their drinking careers, and provides a baseline for similar studies in other twin samples with longer drinking careers.
Future work on remission should examine measured environmental influences at multiple developmental stages in the life course, and also at different stages in the course of alcohol use (regardless of life stage) because genetic and environmental influences may vary as a function of chronological life course or as a function of course of AUD. A broad range of environmental factors should be examined, ranging from socio-economic status to medications taken by one twin but not the other. Generalizability to other samples should also be examined because environmental differences may influence drinking patterns and remission (Rose et al. 1999, 2001a).
In conclusion, the current study highlights the importance of environmental influences on remission and establishes young adult remission as a phenotype distinct from AUD.
Supplementary Material
Acknowledgments
This study was funded by grants AA018146, AA007535, AA017688, AA12640 and AA017921 from the National Institute on Alcoholism and Alcohol Abuse and grant DA14363 from the National Institute on Drug Abuse. We thank the Australian Twin Registry and the twins who participated in this research.
Footnotes
Supplementary Material: For supplementary material accompanying this paper, visit http://dx.doi.org/10.1017/S003329171200044X.
References
- APA. DSM-5 Development: Alcohol Use Disorder. American Psychiatric Association; 2010. [Accessed 14 June 2011]. www.dsm5.org/ProposedRevision/Pages/proposedrevision.aspx?rid=452. [Google Scholar]
- Bischof G, Rumpf HJ, Hapke U, Meyer C, John U. Factors influencing remission from alcohol dependence without formal help in a representative population sample. Addiction. 2001;96:1327–1336. doi: 10.1046/j.1360-0443.2001.969132712.x. [DOI] [PubMed] [Google Scholar]
- Bottlender M, Soyka M. Outpatient alcoholism treatment: predictors of outcome after 3 years. Drug and Alcohol Dependence. 2005;80:83–89. doi: 10.1016/j.drugalcdep.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Charney DA, Zikos E, Gill KJ. Early recovery from alcohol dependence: factors that promote or impede abstinence. Journal of Substance Abuse Treatment. 2010;38:42–50. doi: 10.1016/j.jsat.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Dawson DA. Correlates of past-year status among treated and untreated persons with former alcohol dependence: United States, 1992. Alcoholism: Clinical and Experimental Research. 1996;20:771–779. doi: 10.1111/j.1530-0277.1996.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcoholism: Clinical and Experimental Research. 2007;31:2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS. Maturing out of alcohol dependence: the impact of transitional life events. Journal of Studies on Alcohol. 2006;67:195–203. doi: 10.15288/jsa.2006.67.195. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan WJ. Recovery from DSM-IV alcohol dependence: United States, 2001-2002. Addiction. 2005;100:281–292. doi: 10.1111/j.1360-0443.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Betancourt M, Gilder DA. The clinical course of alcoholism in 243 Mission Indians. American Journal of Psychiatry. 2004;161:1204–1210. doi: 10.1176/appi.ajp.161.7.1204. [DOI] [PubMed] [Google Scholar]
- Gilder DA, Lau P, Corey L, Ehlers CL. Factors associated with remission from alcohol dependence in an American Indian community group. American Journal of Psychiatry. 2008;165:1172–1178. doi: 10.1176/appi.ajp.2008.07081308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JD, Agrawal A, Bucholz KK, Madden PA, Pergadia ML, Nelson EC, Lynskey MT, Todd RD, Todorov AA, Hansell NK, Whitfield JB, Martin NG, Heath AC. Alcohol consumption indices of genetic risk for alcohol dependence. Biological Psychiatry. 2009;66:795–800. doi: 10.1016/j.biopsych.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94:981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Research. 2002;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA – a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LJ. A twin-family study of alcoholism in women. American Journal of Psychiatry. 1994;151:707–715. doi: 10.1176/ajp.151.5.707. [DOI] [PubMed] [Google Scholar]
- Knop J, Penick EC, Jensen P, Nickel EJ, Gabrielli WF, Mednick SA, Schulsinger F. Risk factors that predicted problem drinking in Danish men at age thirty. Journal of Studies on Alcohol. 2003;64:745–755. doi: 10.15288/jsa.2003.64.745. [DOI] [PubMed] [Google Scholar]
- Knop J, Penick EC, Nickel EJ, Mednick SA, Jensen P, Manzardo AM, Gabrielli WF. Paternal alcoholism predicts the occurrence but not the remission of alcoholic drinking: a 40-year follow-up. Acta Psychiatrica Scandinavica. 2007;116:386–393. doi: 10.1111/j.1600-0447.2007.01015.x. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB, Martin NG. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychological Medicine. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, Hasin DS, De Los Cobos JP, Pines A, Wang S, Grant BF, Blanco C. Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addiction. 2011;106:657–669. doi: 10.1111/j.1360-0443.2010.03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, Statham DJ, Martin NG. Escalation of drug use in early-onset cannabis users vs co-twin controls. Journal of the American Medical Association. 2003;289:427–433. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. Department of Psychiatry, Virginia Commonwealth University; Richmond, VA: 2003. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Kluwer Academic; Dordrecht: 1992. [Google Scholar]
- Penick EC, Knop J, Nickel EJ, Jensen P, Manzardo AM, Lykke-Mortensen E, Gabrielli WF. Do premorbid predictors of alcohol dependence also predict the failure to recover from alcoholism? Journal of Studies on Alcohol and Drugs. 2010;71:685–694. doi: 10.15288/jsad.2010.71.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcoholism: Clinical and Experimental Research. 1999;23:1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Hewitt JK, Truett KR, Heath AC, Neale MC, Eaves LJ. Genetic and environmental influences on lifetime alcohol-related problems in a volunteer sample of older twins. Journal of Studies on Alcohol. 1994;55:184–202. doi: 10.15288/jsa.1994.55.184. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. American Journal of Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kuhn JW, Pederson NL. Twin pair resemblance for psychiatric hospitalization in the Swedish Twin Registry: a 32-year follow-up study of 29,602 twin pairs. Behavior Genetics. 2007;37:547–558. doi: 10.1007/s10519-007-9143-6. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcoholism: Clinical and Experimental Research. 2001a;25:637–643. [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Pulkkinen L, Kaprio J. Drinking or abstaining at age 14? A genetic epidemiological study. Alcoholism: Clinical and Experimental Research. 2001b;25:1594–1604. [PubMed] [Google Scholar]
- Rose RJ, Kaprio J, Winter T, Koskenvuo M, Viken RJ. Familial and socioregional environmental effects on abstinence from alcohol at age sixteen. Journal of Studies on Alcohol: Supplement. 1999;13:63–74. doi: 10.15288/jsas.1999.s13.63. [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lynskey MT, Bucholz KK, Madden PA, Martin NG, Heath AC. Timing of first alcohol use and alcohol dependence: evidence of common genetic influences. Addiction. 2009;104:1512–1518. doi: 10.1111/j.1360-0443.2009.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, McCutcheon VV, Pommer NE, Nelson EC, Grant JD, Duncan AE, Waldron M, Bucholz KK, Madden PA, Heath AC. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychological Medicine. 2011;41:1497–505. doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Bucholz KK, Reich T, Bierut L. Five-year clinical course associated with DSM-IV alcohol abuse or dependence in a large group of men and women. American Journal of Psychiatry. 2001;158:1084–1090. doi: 10.1176/appi.ajp.158.7.1084. [DOI] [PubMed] [Google Scholar]
- van Beek JH, Kendler KS, de Moor MH, Geels LM, Bartels M, Vink JM, van den Berg SM, Willemsen G, Boomsma DI. Stable genetic effects on symptoms of alcohol abuse and dependence from adolescence into early adulthood. Behavior Genetics. 2012;42:40–56. doi: 10.1007/s10519-011-9488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viken RJ, Kaprio J, Koskenvuo M, Rose RJ. Longitudinal analyses of the determinants of drinking and of drinking to intoxication in adolescent twins. Behavior Genetics. 1999;29:455–461. doi: 10.1023/a:1021631122461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.