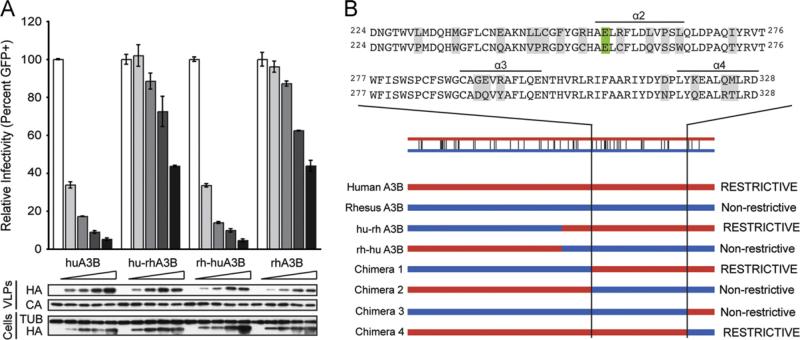
Fig. 1.
HIV-1 restriction potential maps to the human APOBEC3B C-terminal domain. (A) HIV-1 infectivity in the presence of increasing amounts of human APOBEC3B, rhesus APOBEC3B, or the human-rhesus/rhesus-human chimeras. A constant amount of Vif-deficient A200C HIV-1IIIB molecular clone was co-transfected into HEK293T cells with an increasing gradient of the indicated APOBEC3B vector. Percent infectivity of HIV-1IIIB is measured by infection of CEM-GFP in duplicate and flow cytometry, reported as the mean of the two technical replicates ± standard deviation. Representative immunoblots of HA-tagged APOBEC3B proteins (HA) in cell lysates and HIV-1 particles produced by those cells are shown with their respective tubulin (TUB) and p24 (CA) loading controls. (B) Schematic of human/rhesus APOBEC3B C-terminal domain chimeras and their HIV-1 restriction potential. HIV-1 single cycle assays were performed with each chimera as in (A) and found to be restrictive or non-restrictive depending on their similarity to the human or rhesus APOBEC3B parent, respectively. An alignment of human (top) and rhesus (bottom) APOBEC3B amino acid sequences to which the restriction determinant mapped is shown above. Differences between the human and rhesus sequences are highlighted in gray and the catalytic glutamate is highlighted in green. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)