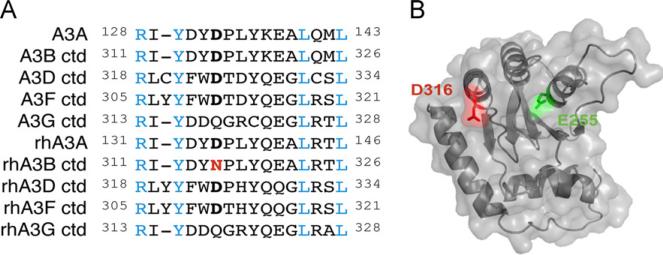
Fig. 4.
Conservation and predicted structural context of APOBEC3B D316. (A) Amino acid sequence alignment of the predicted loop 7 regions of human and rhesus APOBEC3B and their related family members. Blue residues indicate perfect conservation. APOBEC3B residue 316 and its predicted homologs are bolded in black (aspartic acid) or red (asparagine). (B) Predicted structure of human APOBEC3B C-terminal domain based on the human APOBEC3G C-terminal domain crystal structure (PDB: 3IR2). D316 is highlighted in red in close proximity to the catalytic E255 in green. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)