TABLE 1.
Screening of olefination conditions
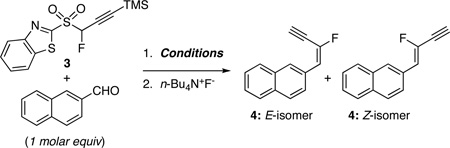 | |||||
|---|---|---|---|---|---|
| entry | basea | solvent | Additive | T (°C); time | (%) E/Z;b yield (%)c |
| 1d | KHMDS | THF | -- | −55; 10 min | 82/18; 86 |
| 2d | LHMDS | THF | -- | −78; 10 min | 88/12; 97 |
| 3d | LHMDS | THF | MgBr2Et2Oe | rt; 4 h | 89/11; 78 |
| 4d | LHMDS | DMF | DMPUf | −78; 10 min | 63/37; 74 |
| 5d | LHMDS | DMPU | -- | 0; 10 min | 58/42; NAg |
| 6h | DBU | CH2Cl2 | -- | rt; 10 min | 69/31; 91 |
| 7h | DBU | CH2Cl2 | -- | 0; 10 min | 70/30; 92 |
| 8h | DBU | CH2Cl2 | -- | −55; 10 min | 74/26; 95 |
| 9h | DBU | CH2Cl2 | -- | −78; 10 min | 74/26; NAg |
| 10h | DBU | CH2Cl2 | MgBr2Et2Oe | rt; 30 h | 60/40; Inci |
| 11h | DBU | THF | MgBr2Et2Oe | rt; 30 h | 90/10; Inci |
| 12h | Cs2CO3 | CH2Cl2 | -- | rt; 6 h | 74/26; NAg |
KHMDS and LHMDS: 2.4 molar equiv. DBU: 4.0 molar equiv. Cs2CO3: 3.0 molar equiv.
E/Z ratio of diastereomers in the crude reaction mixture was determined by 19F NMR, prior to isolation.
Yields are of isolated and purified products.
Sulfone 3, 2.0 molar equiv.
3.0 molar equiv.
Cosolvent, ratio of DMF/DMPU 1:1 (v/v).
Products were not isolated.
Sulfone 3, 2.5 molar equiv.
Reaction was incomplete after 30 h.
