TABLE 2.
Condensation reactions of 3 with aldehydes and ketones
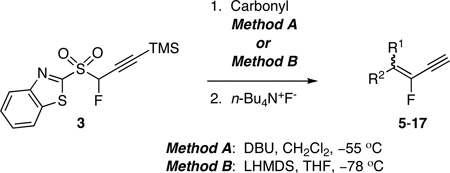 | |||
|---|---|---|---|
| product: yield,a E/Zb | |||
| entry | carbonyl | method Ac | method Bd |
| 1 | 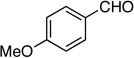 |
5: 92%, 81/19 | 5: 97%, 95/5 |
| 2 | 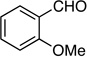 |
6: 92%, 78/22 | 6: 87%, 90/10 |
| 3 | 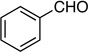 |
7: 90%, 76/24 | 7: 59%, 94/6 |
| 4 | 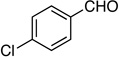 |
8: 81%, 70/30 | 8: 95%, 94/6 |
| 5 | 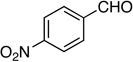 |
9: 59%, 51/49 | 9: 81%, 72/28 |
| 6 | 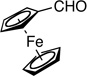 |
10: 95%, 83/17 | 10: 98%, 90/10 |
| 7 | 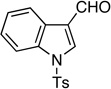 |
11: 95%, 75/25 | 11: 92%, 91/9 |
| 8 | 12: 78%, 75/25 | 12: 78%, 91/9 | |
| 9 | 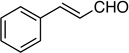 |
13: 95%, 72/28 | 13: 96%, 80/20 |
| 10 | 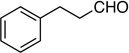 |
14: 54% (63%e), 80/20 |
14: 63%, 70/30 |
| 11 |
15: 79% (91%e), 85/15 |
-- | |
| 12 | 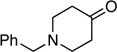 |
-- | 16: 77% |
| 13 | 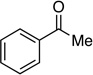 |
-- | 17: 88%, E onlyf |
Yields of isolated and purified products.
E/Z ratio of diastereomers in the crude reaction mixture was determined by 19F NMR, prior to isolation. No change in ratio was observed after purification.
Method A: sulfone 3, 2.0–2.5 molar equiv; DBU, 4.0 molar equiv; TBAF, 0.20 molar equiv. For 11 and 13, 3.0 molar equiv of 3 was used. Sulfone was added to solution of aldehyde and base.
Method B for aldehydes: sulfone 3, 2.0 molar equiv; LHMDS, 2.4 molar equiv; TBAF, 2.0 molar equiv. Method B for ketones: sulfone 3, 3.0 molar equiv; LHMDS, 5.0 molar equiv; TBAF, 3.0 molar equiv. LHMDS was added to a solution of sulfone and aldehyde.
Yield was calculated using octafluoronaphthalene as an internal standard.
Determined by NOESY experiment on the diene obtained by Lindlar reduction of 17 (see Figure 5).
