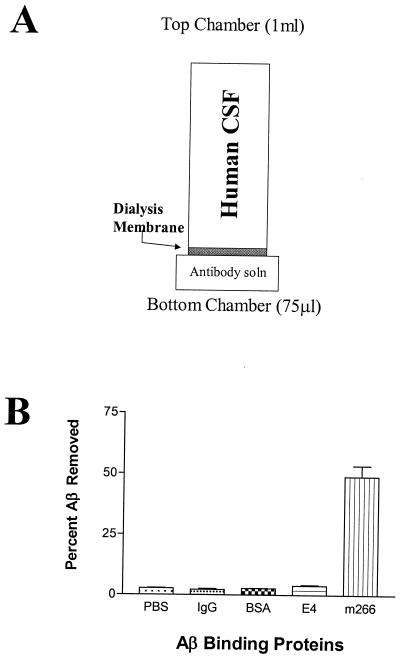
Figure 1.
Anti-Aβ antibody m266 acts as an Aβ sink. (A) An in vitro assay was developed to identify the relative efficiency of Aβ binding proteins on sequestering soluble CNS Aβ. One milliliter of human CSF was placed in the top chamber of a tube separated by a 25-kDa dialysis membrane from a bottom chamber that contained 75 μl of PBS. Human CSF used in these studies contained, on average, 10 ng/ml of AβTotal (2.5 pmol/ml). (B) The % Aβ removed from the top chamber was determined by Aβ ELISA analysis of both the top and bottom chamber (n = 4 per condition) after a 3-h incubation at 37°C. The bottom chamber contained PBS ± 20 μg of the listed proteins (IgG and m266, 133 pmol; BSA, 332 pmol; apoE4, 585 pmol). Affinity-purified, astrocyte-secreted apoE4 lipoproteins, a known Aβ binding protein, sequestered significantly more Aβ (3.86%, P < 0.05) to the bottom chamber than nonspecific mouse IgG (2.18%) or BSA (2.64%). The Aβ antibody m266 was dramatically more efficient at sequestering CSF Aβ (48.91%) to the bottom chamber as compared with all other conditions tested (P < 0.001). Data were analyzed with ANOVA followed by post hoc Tukey's test.