Figure 8.
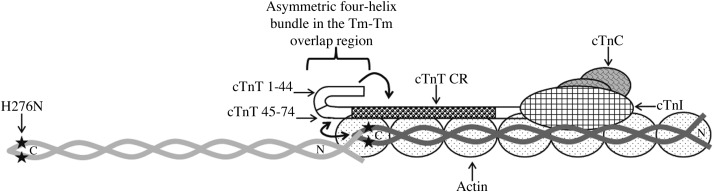
Schematic representation of the asymmetric 4-helix bundle in the head-to-tail overlapping region of 2 contiguous Tms. The 4-helix bundle (2 helices from the C termini of Tm, one from the N terminus of Tm, and one from the central region of cTnT) results from local disruption of coiled-coil regions in the N terminus of Tm (11). The precise configuration of this molecular swivel structure is considered to be important for the flexibility of Tm on actin filaments. Flexibility not only enables adjacent Tm to wind around actin properly, but it also permits the filamentous Tm to occupy discrete positions on actin during thin-filament activation and deactivation. The H276N mutation in the C terminus of Tm is denoted by black stars. The central helical region of cTnT (residues 78–193) is represented by CR. Amino acid residues 1–77 of cTnT are not known to interact with Tm, actin, TnC, or TnI; however, this region is known to synergistically modulate the ability of CR to affect Tm function (shown by single-headed arrow; Gollapudi et al., ref, 37). The intermolecular interaction between the N terminus of cTnT and the overlapping ends of Tm is denoted by the double-headed arrow. (Adapted from Gollapudi et al., ref. 37).
