Figure 1.
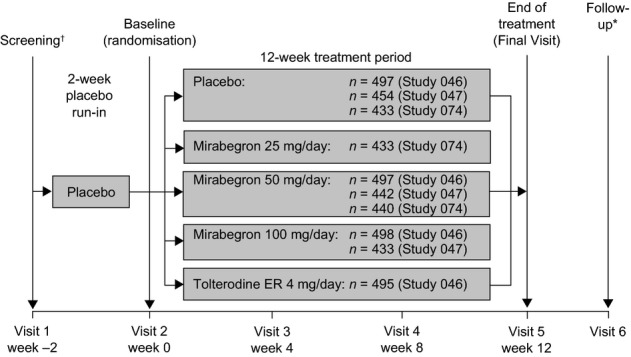
Overview of the individual phase III studies included in the pooled analyses. †Screening from weeks −3 to −2; *Evaluation of adverse events and concomitant medication by telephone contact or visit 30 days after Final Visit in studies 046 and 047 and 2 weeks after Final Visit in Study 074. n, number of patients randomised
