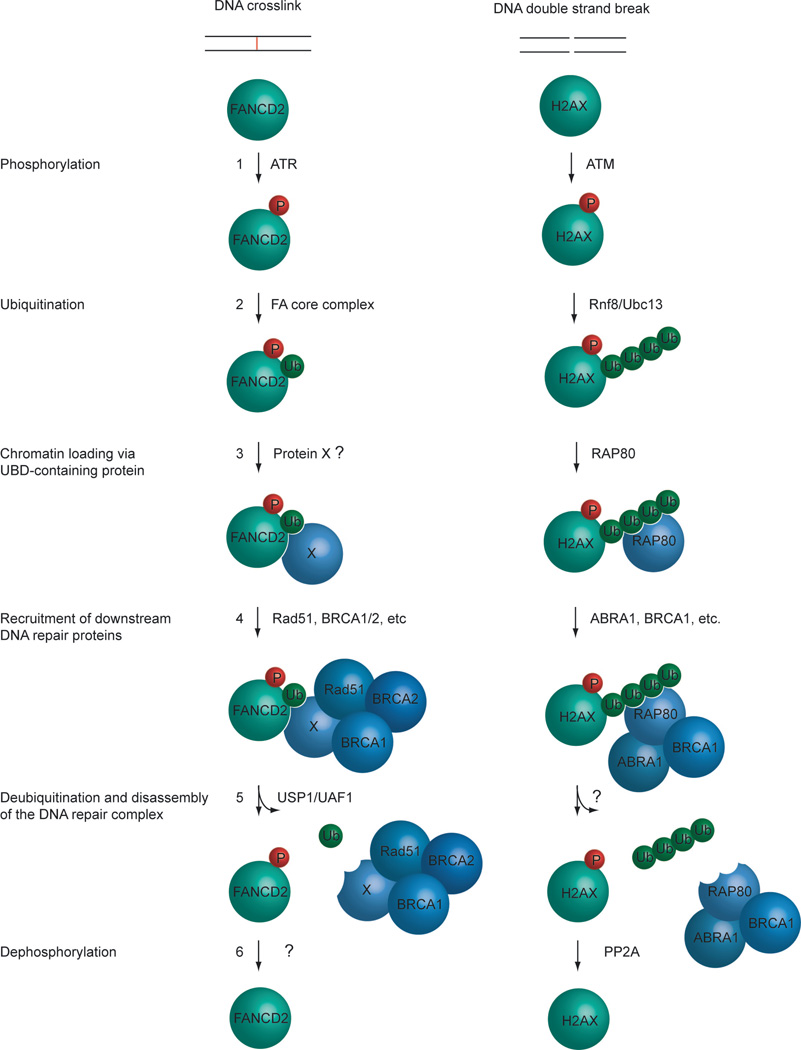
Figure 2. Conserved elements of the Fanconi Anemia and DSB repair pathways.
1) In response to either a DNA crosslink or double strand break, ATR or ATM phosphorylates FANCD2 or H2AX, respectively. 2) The phosphorylated proteins are now substrates for a ubiquitination event, carried out either by the FA core complex or the RNF8/UBC13 complex. 3) Monoubiquitinated FANCD2 protein can now be recruited to sites of damage on the chromatin by a recruitment factor, Protein X. Polyubiquitinated H2AX recruits RAP80 to sites of damage on the chromatin. 4) The properly localized FANCD2 protein associates with other DNA repair proteins and the DNA damage is repaired. In addition to RAP80, other DNA repair proteins are recruited to H2AX, and the DNA damage is repaired. 5) Once the DNA repair process has been completed, the recruited protein complex must be disassembled. This is achieved by deubiquitinating the FANCD2 protein by the USP1/UAF1 complex, and H2AX by an unknown deubiquitinating enzyme. 6) After the DNA repair process has been completed, FANCD2 is dephosphorylated by an unknown phosphatase, and H2AX is dephosphorylated by PP2A (Chowdhury et al., 2005). For simplicity, this model only shows a subset of the proteins known to participate in ICL and DSB repair. For instance, only FANCD2 is shown, although the paralog protein, FANCI, is believed to act in a similar fashion.