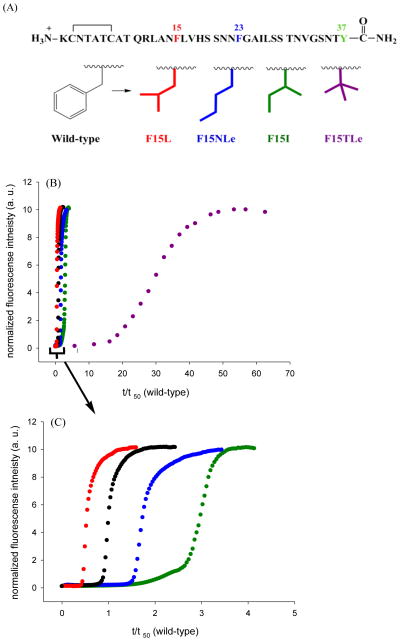
Figure 6.
The rate of amyloid formation is affected by substitution at position 15, but there is no correlation with β-sheet propensity. (A) A series of varients with isomeric four-carbon side chains were analyzed. These “mutations” change α-helix propensity and β-sheet propensity, but maintain hydrophobicity. (B) Thioflavin-T fluorescence monitored kinetic experiments are shown. Black, wild-type IAPP; red, F15L-IAPP; blue, F15NLe-IAPP; green, F15I-IAPP; purple, F15TLe-IAPP. (C) An enlarged plot covering the range from t/t50 (wild-type) = 0 to 5.