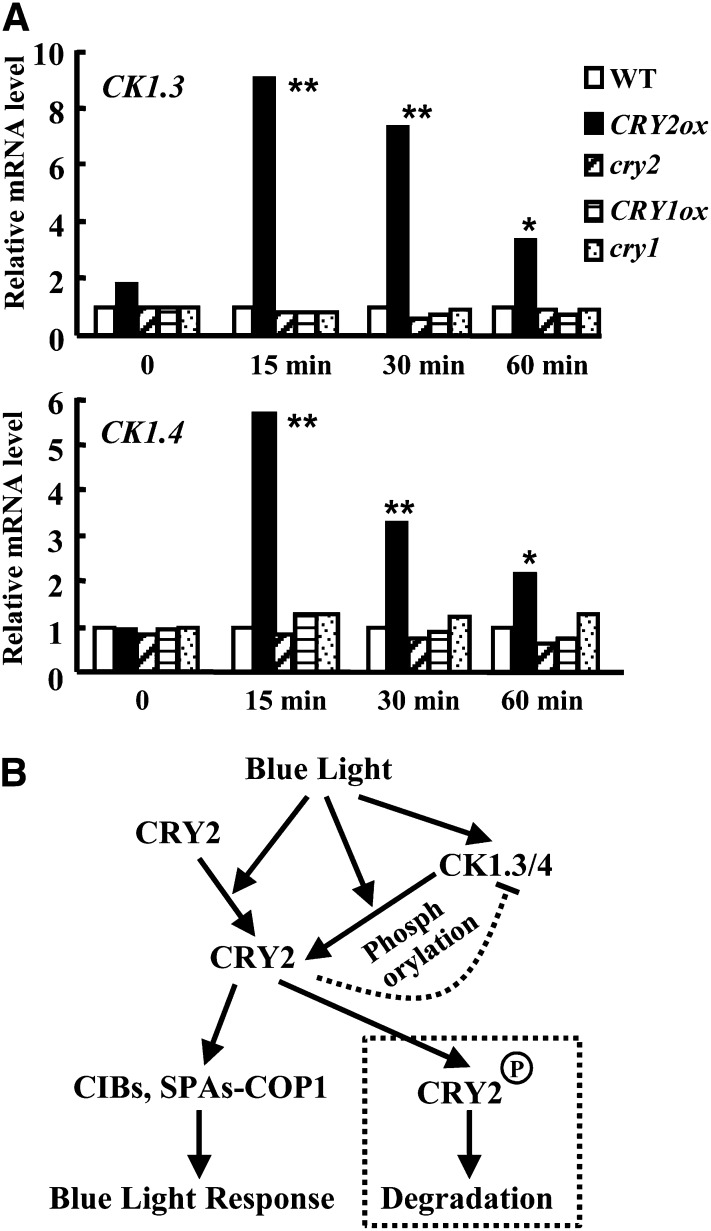
Figure 8.
Expression of CK1.3 and CK1.4 Is Mediated by CRY2 and a Hypothetical Model of How CK1s Are Involved in CRY2-Mediated Blue Light Signaling.
(A) qRT-PCR analysis revealed that the expression of CK1.3 (top panel) or CK1.4 (bottom panel) is rapidly induced under blue light (3 µmol/m2/s fluence rate) for 15, 30, and 60 min in a CRY2 overexpression background. In addition, expression of CK1.3 and CK1.4 were reduced in cry2 mutant, and there was no significant change in either cry1 mutant or CRY1ox (CK1.4 expression was reduced in cry1). ACTIN7 was used as a positive internal reference. Expression of CK1.3 or CK1.4 in the wild type (WT) under blue light condition (3 µmol/m2/s fluence rate) for 0, 15, 30, and 60 min was set as 1.0. Statistical analysis indicated the significant differences (*P < 0.05; **P < 0.01). The experiments were repeated three times.
(B) Hypothetical model of the role of CK1.3 and CK1.4 in CRY2-mediated blue light signaling. As an important blue light receptor, the resting CRY2 under dark is activated by blue light and undergoes conformational changes to expand the active motif to bind downstream partners, including CIBs and SUPPRESSOR OF PHYTOCHROME As-CONSTITUTIVE PHOTOMORPHOGENIC1, to confer the blue light responses. At the same time, expression of CK1.3 and CK1.4 is rapidly induced in a CRY2-dependent manner. The conformational change of CRY2 may expose the candidate phosphorylation sites, Ser-587 and Thr-603, recognized by CK1.3 and CK1.4, which results in blue light–stimulated CRY2 degradation through phosphorylation. The exquisite regulation of expression of CK1s and CRY2 degradation reveals the complex and finely controlled blue light signaling.