Figure 2.
CURT1 Proteins Are Intrinsic Thylakoid Proteins.
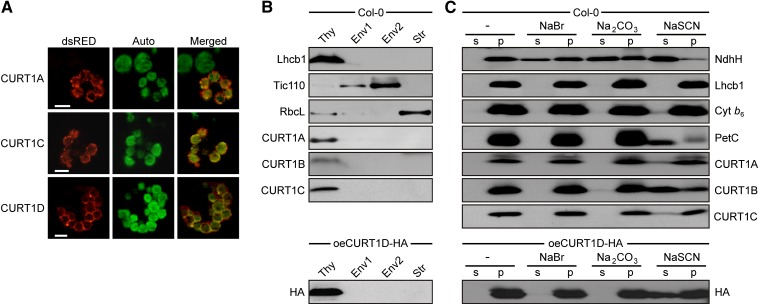
(A) Subcellular localization of CURT1 proteins. Full-length CURT1A-, CURT1C-, and CURT1D-dsRED fusions were transiently introduced into Arabidopsis protoplasts by polyethylene glycol–mediated DNA uptake and analyzed using fluorescence microscopy (Auto, chloroplasts revealed by chlorophyll autofluorescence; dsRED, fluorescence of the fusion protein; Merged, overlay of both images). Bar = 10 μm.
(B) Suborganellar localization of CURT1 proteins. Chloroplasts from wild-type and oeCURT1D-HA plants were subfractionated into thylakoids (Thy), stroma (Str), and two envelope (Env) fractions. Aliquots (40 µg) of protein from each fraction were subjected to SDS-PAGE, followed by immunoblot analysis using antibodies raised against CURT1A, B, C, or HA. As controls for purity of the different fractions, antibodies recognizing Lhcb1, Tic110, and RbcL, which are located in thylakoids, envelope, and stroma, respectively, were used.
(C) Extraction of thylakoid-associated proteins with chaotropic salt solutions or alkaline pH. Thylakoids from wild-type and oeCURT1D-HA plants were resuspended at 0.5 mg chlorophyll/mL in 10 mM HEPES/KOH, pH 7.5, containing either 2 M NaBr, 0.1 M Na2CO3, 2 M NaSCN, or no additive. After incubation, supernatants containing the extracted proteins (s) and membrane fractions (p) were separated by SDS-PAGE and immunolabeled with antibodies against CURT1A, B, C, and HA. As control for peripheral membrane proteins, antibodies raised against NdhH were used. To control for integral membrane proteins, antibodies specific for Lhcb1, cytochrome b6, and Rieske (PetC) were employed.