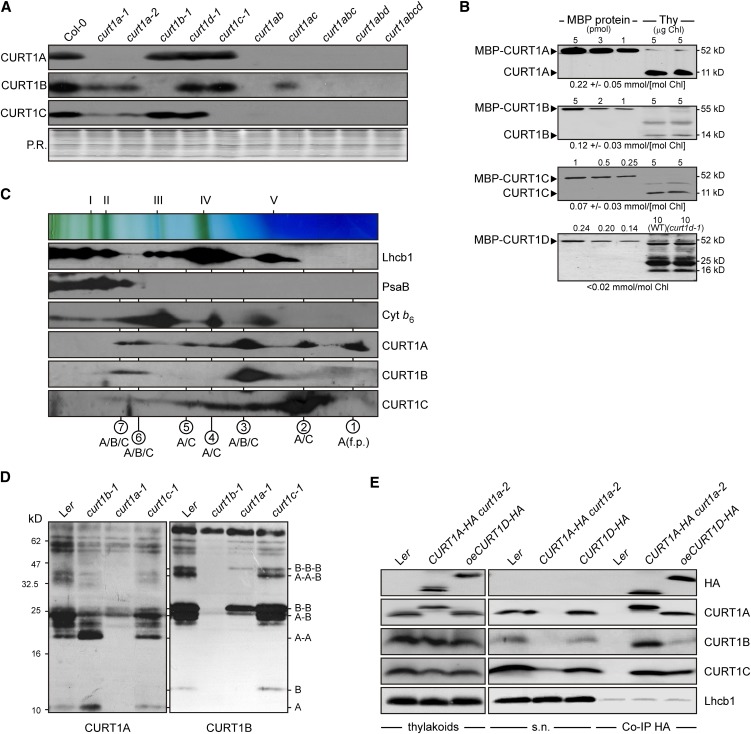
Figure 3.
CURT1 Proteins Form Oligomers.
(A) Abundance of CURT1 proteins in curt1 mutant plants. Total protein extracts from Col-0 and curt1 mutant plants corresponding to 3 µg of chlorophyll were fractionated by SDS-PAGE, and blots were probed with antibodies raised against CURT1A, B, and C. The Ponceau Red (P.R.)–stained protein blot served as loading control.
(B) Absolute abundance of CURT1 proteins in wild-type (WT) plants. The four Arabidopsis CURT1 proteins were expressed in Escherichia coli as C-terminal fusions to the MBP, purified by affinity chromatography, and quantified. Adequate quantities of these four MBP fusions, as well as of the MBP fusions of PetC and PsaD as controls (see Supplemental Figure 3E online), were titrated against thylakoid membrane protein preparations and subjected to immunoblot analyses. Representative results from five experiments are shown. The calculated concentration of the respective CURT1 protein in thylakoid preparations is given below each panel.
(C) Two-dimensional BN/SDS-PAGE separation of thylakoid protein complexes. Individual lanes from BN-PA gels like those shown on top were separated in a second dimension by SDS-PAGE. Blots were immunolabeled with antibodies raised against Lhcb1, PsaB, cytochrome b6, and CURT1A, B, and C. The positions of major thylakoid multiprotein complexes are indicated by Roman numerals (top) and the composition of the complexes by circled Arabic numbers (bottom) as in Supplemental Figure 3F online. f.p., free protein.
(D) Chemical cross-linking. Thylakoid proteins from wild-type (Landsberg erecta [Ler]) and mutant (curt1b-1, curt1a-1, and curt1c-1) plants were cross-linked with bis(sulfosuccinimidyl) suberate, separated by SDS-PAGE, and subjected to immunoblot analysis with antibodies raised against CURT1A and B. On the right, the protein composition of cross-linked products is shown. As loading control, blots were stained with Ponceau Red (see Supplemental Figure 3H online).
(E) CoIP. Thylakoid membranes from wild-type, CURT1A-HA curt1a-2, and oeCURT1D-HA plants were solubilized, and HA-tagged proteins were allowed to bind to α-HA affinity matrix. The matrix was recovered and the supernatant was collected. After washing, coimmunoprecipitated proteins were eluted. Thylakoids, supernatant (s.n.), and coimmunoprecipitated proteins (Co-IP HA) were then analyzed by SDS-PAGE followed by immunoblot analysis with antibodies raised against HA, CURT1A, B, and C, and Lhcb1.