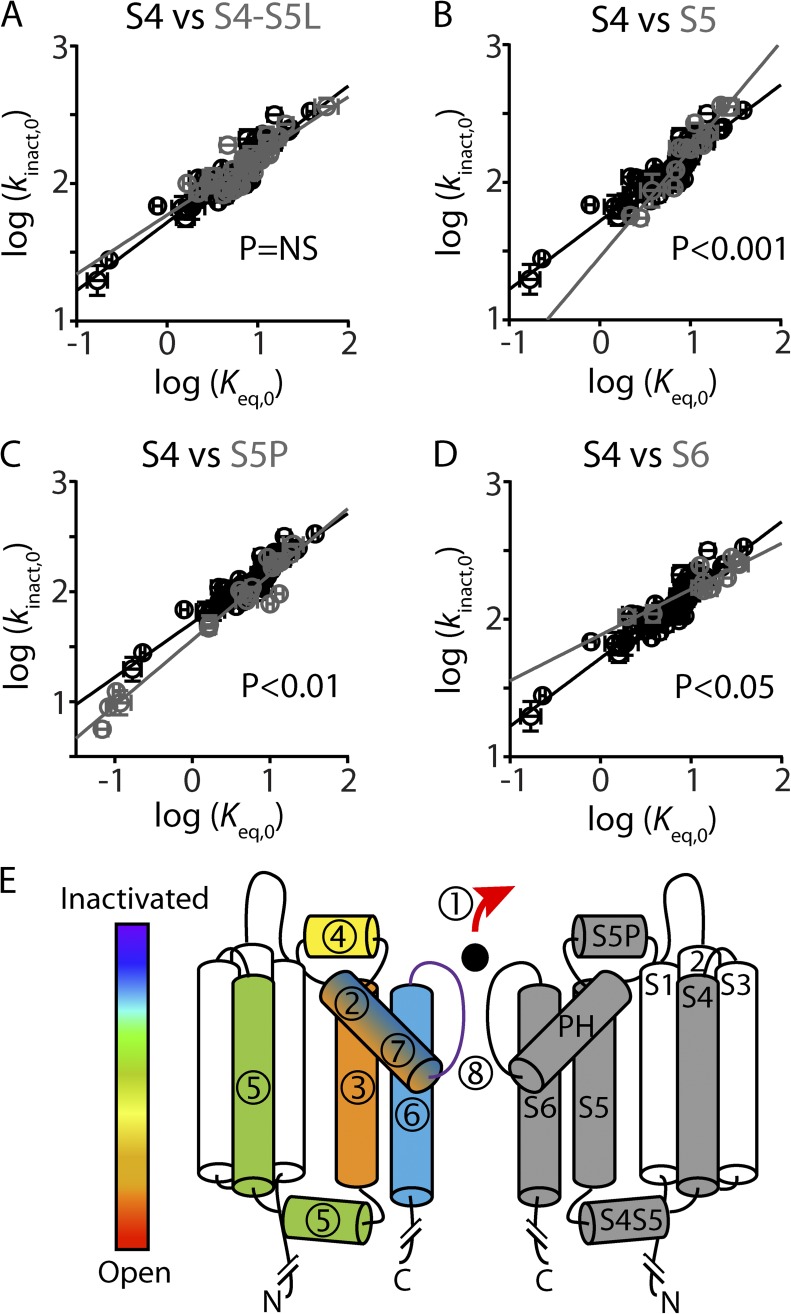
Figure 8.
Multi-domain model of inactivation gating in Kv11.1 channels. (A–D) REFER plots to compare S4 residue mutations (shown in black) against S4S5 linker (A), S5 (B), S5P linker (C), and S6 (D) residue mutations (shown in gray). ANCOVA shows that the linear regression slope, which represents the Φ-value, was significantly different for the S4 helix compared with the S5, S5P, and S6 helices (P < 0.05), but not the S4–S5 linker. (E) Cartoon showing two opposing subunits of Kv11.1. Segments in the right-hand subunit are labeled S1–S6 and pore helix (PH). Segments in the left-hand subunit are color and number coded, according to derived Φ-values (present study and Wang et al., 2011; Perry et al., 2013), to show the relative sequence of events that occur during the open-to-inactivated gating transition, where red (1) indicates the first step and purple (8) indicates the putative final step. The S4 helix (green, 5), as well as the S4–S5 linker, experience a change in environment shortly after the S5 helix and S5P linker but before the S6 helix. We propose that hydrophobic interactions between the S4 helix and the S5 helix help couple the voltage-sensor domain to the pore domain during the open-to-inactivated gating transition of Kv11.1 channels.