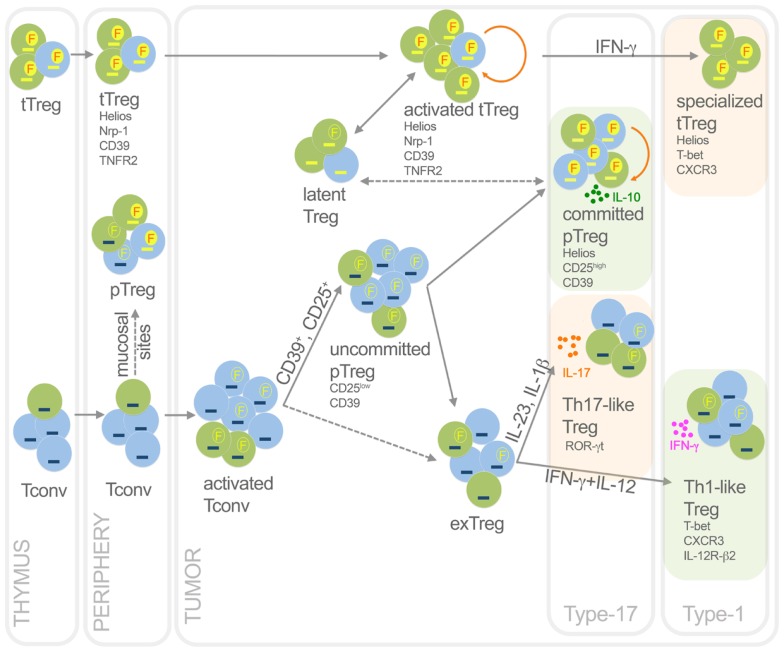
Figure 2.
Functional dynamics of tTreg and pTreg in cancer. This picture summarizes development, heterogeneity, plasticity, antigen specificity, and function of pTreg and tTreg in cancer. Activated Treg, which are epigenetically committed and mostly self- and TAA-specific, can transiently lose Foxp3 without methylating TSDR thus becoming latent Treg; in some conditions, they can acquire T-bet expression thus becoming specialized suppressors, detrimental to the anti-tumor type-1 response. Activated Tconv, mostly foreign (TSA) antigen-specific, can promiscuously express Foxp3 without demethylating TSDR. However, a fraction (CD25+, or CD39+) of activated Tconv can convert into pTreg, progressively moving from an uncommitted to a committed stage. Through IL-10, committed pTreg can suppress pro-tumoral inflammatory and type-17 responses, thus exerting beneficial roles for the host in some cancer types. In some contexts, uncommitted pTreg (and possibly activated Tconv) can move back to exTreg stage, acquiring the ability to produce inflammatory cytokines. Therefore, in some tumors such as colon cancer, Th17-like Treg may foster type-17 inflammation thus supporting tumor growth; in other tumor contexts, Th1-like Treg can favor type-1 responses that rather block tumor growth. Green, cells specific for self-antigens and TAA; light blue, cells specific for foreign antigens including TSA. Yellow dash, demethylated TSDR; blue dash, methylated TSDR. Red “F” in yellow circles, stable Foxp3; yellow “F” in empty circles, unstable Foxp3. Dashed arrows, unclear events. Orange rounded arrows, proliferation in the tTreg or the pTreg homeostatic niche. Light green frames, conditions in which Treg are beneficial to the host; light orange frames, conditions in which Treg are detrimental to the host.