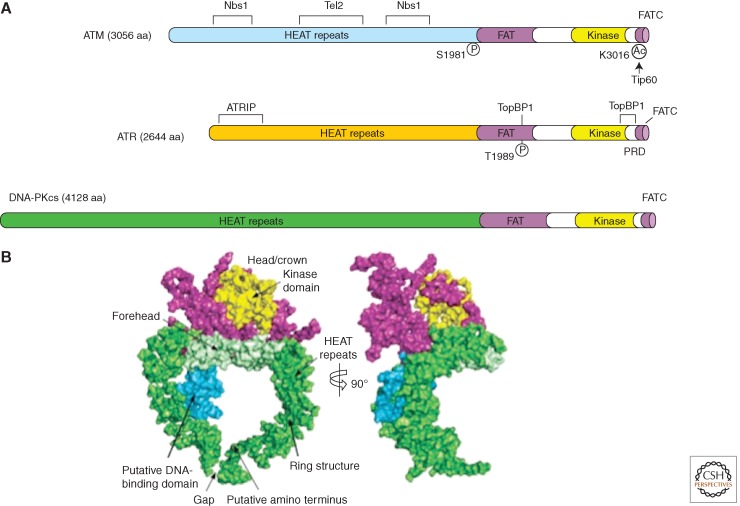
Figure 2.
Structural outlines of PIKKs. (A) Schematic of the functional domains and selected posttranslational modifications of ATM, ATR, and DNA-PKcs. The bulk of ATM and ATR are composed of a large number (40–50) of α-helical HEAT repeats. Some of these repeats are involved in the interaction with other proteins. The kinase domains of ATM and ATR are located near their carboxyl termini, and are flanked by FAT and FATC domains. ATM and ATR are autophosphorylated in or near their FAT domains after DNA damage, which regulates their activation. The acetylation of the carboxyl terminus of ATM by Tip60 is also involved in ATM activation. A PIKK regulatory domain (PRD) between the kinase and FATC domains of ATR was shown to modulate ATR activation. (B) Overall view of the DNA-PKcs structure. The front view (left) and side view (right) of DNA-PKcs. The various regions of DNA-PKcs are colored and labeled. (Panel B is from Sibanda et al. 2009; reprinted, with permission, from Nature Publishing Group © 2009.)