Abstract
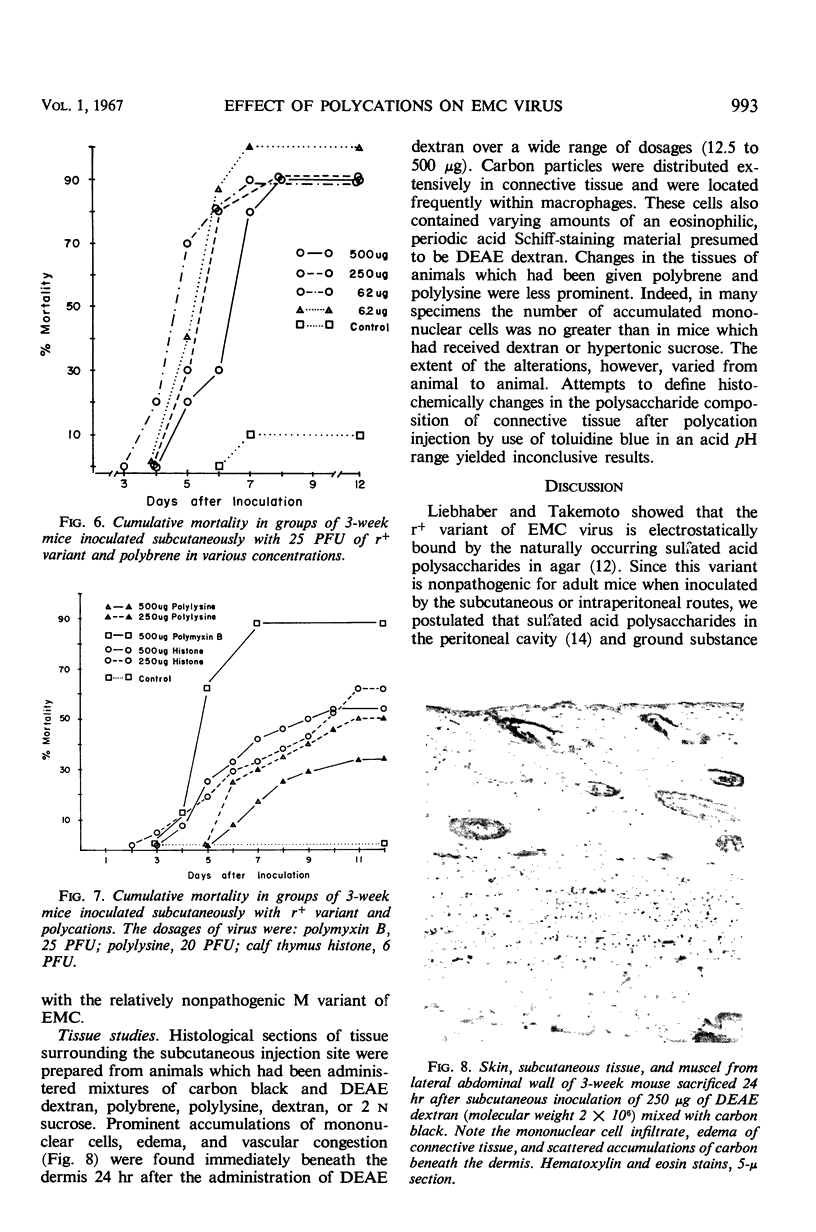
Pathogenicity of the encephalomyocarditis (EMC) virus for adult mice was increased when polycations of diverse type were mixed with virus and inoculated by the subcutaneous or intraperitoneal routes. Diethylaminoethyl (DEAE) dextran, hexadimethrine (polybrene), polymyxin B, polylysine, and calf thymus histone in various concentrations stimulated multiplication of virus in tissues at the injection site and enhanced entry of virus into the blood. The nonpathogenic r+ variant of EMC which grows locally in tissues but fails to disseminate after subcutaneous and intraperitoneal inoculation was used in most experiments. This virus caused viremia and fatal central nervous system disease only when the polycations were included in the inoculum. DEAE dextran and polybrene stimulated the release of interferon in infected tissues but had no effect in the absence of virus multiplication. Histological studies of tissues from the injection site showed that polycations provoke a mononuclear cell reaction and alter the integrity of connective tissue. However, the mechanism by which the substances enhance virus growth and dissemination was not defined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARON S., BUCKLER C. E. CIRCULATING INTERFERON IN MICE AFTER INTRAVENOUS INJECTION OF VIRUS. Science. 1963 Sep 13;141(3585):1061–1063. doi: 10.1126/science.141.3585.1061. [DOI] [PubMed] [Google Scholar]
- COLTER J. S., DAVIES M. A., CAMPBELL J. B. STUDIES OF THREE VARIANTS OF MENGO ENCEPHALOMYELITIS VIRUS. II. INHIBITION OF INTERACTION WITH L CELLS BY AN AGAR INHIBITOR AND BY PROTAMINE. Virology. 1964 Dec;24:578–585. doi: 10.1016/0042-6822(64)90210-7. [DOI] [PubMed] [Google Scholar]
- Craighead J. E. Pathogenicity of the M and E variants of the encephalomyocarditis (EMC) virus. I. Myocardiotropic and neurotropic properties. Am J Pathol. 1966 Feb;48(2):333–345. [PMC free article] [PubMed] [Google Scholar]
- Craighead J. E. Polycation effect on virulence of nonpathogenic r+ variant of the encephalomyocarditis virus. Nature. 1967 May 13;214(5089):716–717. doi: 10.1038/214716a0. [DOI] [PubMed] [Google Scholar]
- DE VRIES A., SALGO J., MATOTH Y., NEVO A., KATCHALSKI E. Effect of basic polyamino acids on phagocytosis in vitro. Arch Int Pharmacodyn Ther. 1955 Nov 1;104(1):1–10. [PubMed] [Google Scholar]
- GLASGOW L. A., HABEL K. Interferon production by mouse leukocytes in vitro and in vivo. J Exp Med. 1963 Jan 1;117:149–160. doi: 10.1084/jem.117.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMURA E. T., YOUNG P. R., BARLOW G. H. A study with low and high molecular weights of hexadimethrine bromide-an antiheparin agent. Proc Soc Exp Biol Med. 1962 Oct;111:37–41. doi: 10.3181/00379727-111-27698. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Maes R. F., Campbell J. B., Koprowski H. Effect of polyions on the infectivity of rabies virus in tissue culture: construction of a single-cycle growth curve. J Virol. 1967 Feb;1(1):145–151. doi: 10.1128/jvi.1.1.145-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt W. J., Murphy E. B. Investigations on interferon induced by statolon. Virology. 1965 Dec;27(4):484–489. doi: 10.1016/0042-6822(65)90173-x. [DOI] [PubMed] [Google Scholar]
- LIEBHABER H., TAKEMOTO K. K. Alteration plaque morphology of EMC virus with polycations. Virology. 1961 Aug;14:502–504. doi: 10.1016/0042-6822(61)90349-x. [DOI] [PubMed] [Google Scholar]
- LIEBHABER H., TAKEMOTO K. K. THE BASIS FOR THE SIZE DIFFERENCES IN PLAQUES PRODUCED BY VARIANTS OF ENCEPHALOMYOCARDITIS (EMC) VIRUS. Virology. 1963 Aug;20:559–566. doi: 10.1016/0042-6822(63)90280-0. [DOI] [PubMed] [Google Scholar]
- LOEWI G., MEYER K. The acid mucopolysaccharides of embryonic skin. Biochim Biophys Acta. 1958 Mar;27(3):453–456. doi: 10.1016/0006-3002(58)90371-8. [DOI] [PubMed] [Google Scholar]
- MERGENTHALER D. D., PAFF G. H. Peritoneal mast cells as a possible source of circulating heparin in the rat. Anat Rec. 1956 Oct;126(2):165–175. doi: 10.1002/ar.1091260204. [DOI] [PubMed] [Google Scholar]
- Muir H. Chemistry and metabolism of connective tissue glycosaminoglycans (mucopolysaccharides). Int Rev Connect Tissue Res. 1964;2:101–154. doi: 10.1016/b978-1-4831-6751-0.50009-4. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., Vaheri A. Enhancement of infectivity of poliovirus RNA with diethylaminoethyl-dextran (DEAE-D). Arch Gesamte Virusforsch. 1965;17(3):456–464. doi: 10.1007/BF01241201. [DOI] [PubMed] [Google Scholar]
- Ryser H. J., Hancock R. Histones and basic polyamino acids stimulate the uptake of albumin by tumor cells in culture. Science. 1965 Oct 22;150(3695):501–503. doi: 10.1126/science.150.3695.501. [DOI] [PubMed] [Google Scholar]
- Ryser H. J. Studies on protein uptake by isolated tumor cells. 3. Apparent stimulations due to pH, hypertonicity, polycations, or dehydration and their relation to the enhanced penetration of infectious nucleic acids. J Cell Biol. 1967 Mar;32(3):737–750. doi: 10.1083/jcb.32.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMULL C. E., LUDWIG E. H. Enhancement of the plaque-forming capacity of poliovirus ribonucleic acid with basic proteins. J Bacteriol. 1962 Nov;84:1035–1040. doi: 10.1128/jb.84.5.1035-1040.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMOTO K. K., LIEBHABER H. Virus-polysaccharide interactions. I. An agar polysaccharide determining plaque morphology of EMC virus. Virology. 1961 Aug;14:456–462. doi: 10.1016/0042-6822(61)90338-5. [DOI] [PubMed] [Google Scholar]