Figure 2.
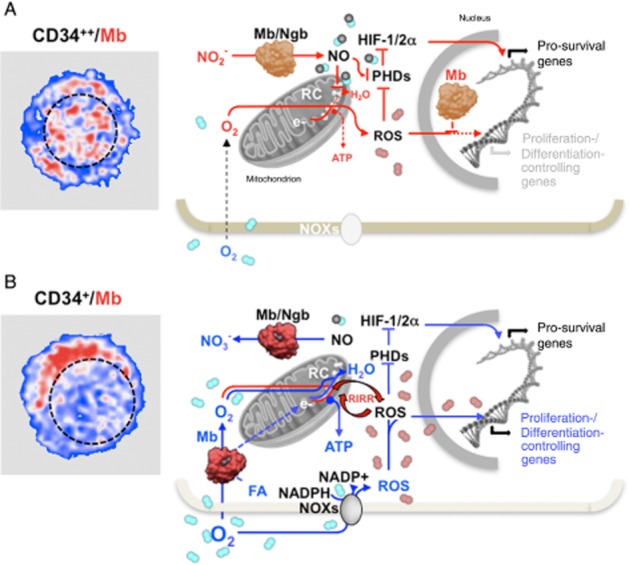
Schematic view of the suggested functional role of globins under hypoxic and normoxic conditions in quiescent and early committed HSPCs. The pictures on the left are artificial colour images of CD34++ (A) and CD34+ (B) in human HSPCs immunostained with a myoglobin (Mb)-Ab. The Mb-related fluorescence signal is shown in red and the nuclear compartment outlined with a dashed black line. The schemes on the right illustrate the suggested functions of Mb and neuroglobin (Ngb) in the context of hypoxic (A) and normoxic (B) conditions in early and late HSPCs respectively. The prevailing de-oxygenated and oxygenated state of the globins is highlighted with a different colour tone in (A) and (B). It is shown that under hypoxia (A) the de-oxygenated form of Mb/Ngb function as a nitrite reductase generating NO. This inhibits the mitochondrial respiratory chain (RC) and the oxidative phosphorylation promoting electron leak to O2 with formation of ROS, which, along with NO, inhibit the prolyl-hydroxylases (PHDs) thereby stabilizing the hypoxia inducible factors (HIF-1/2α). The transcriptional activity of HIF induces the expression of a set of pro-survival genes. The nuclear localization of Mb under this condition prevents ROS from scavenging the redox-linked activation of NFs/genes involved in the proliferation/differentiation of HSPCs or oxidative damage of DNA. Under normoxic conditions (B) the oxygenated form of the globins converts NO in nitrate unleashing the NO inhibition of the RC. This restores mitochondrial O2 consumption and ATP production by oxidative phosphorylation. Under these conditions the globins might favour the delivery of O2 and/or fatty acids (FA) to the mitochondria. It is further shown that the normoxic condition promotes the activity of the NADPH oxidases (NOXs) with the formation of ROS. The ROS level is suggested to be eventually increased by means of a ROS-induced ROS release (RIRR) mechanism involving the mitochondrial RC. The consequent enhanced pro-oxidative state of the cell functions as a signal inducing the HSPC to differentiate. The protective role of the nuclear Mb under this condition is removed by its cytoplasmic re-localization. Depending on the prevailing conditions, set by extrinsic signals, ROS can also activate under normoxic conditions HIF thereby delaying differentiation of HSPCs if these are mobilized to restore exhausted niches or act at hypoxia-injured tissues. See text for further considerations.
