Abstract
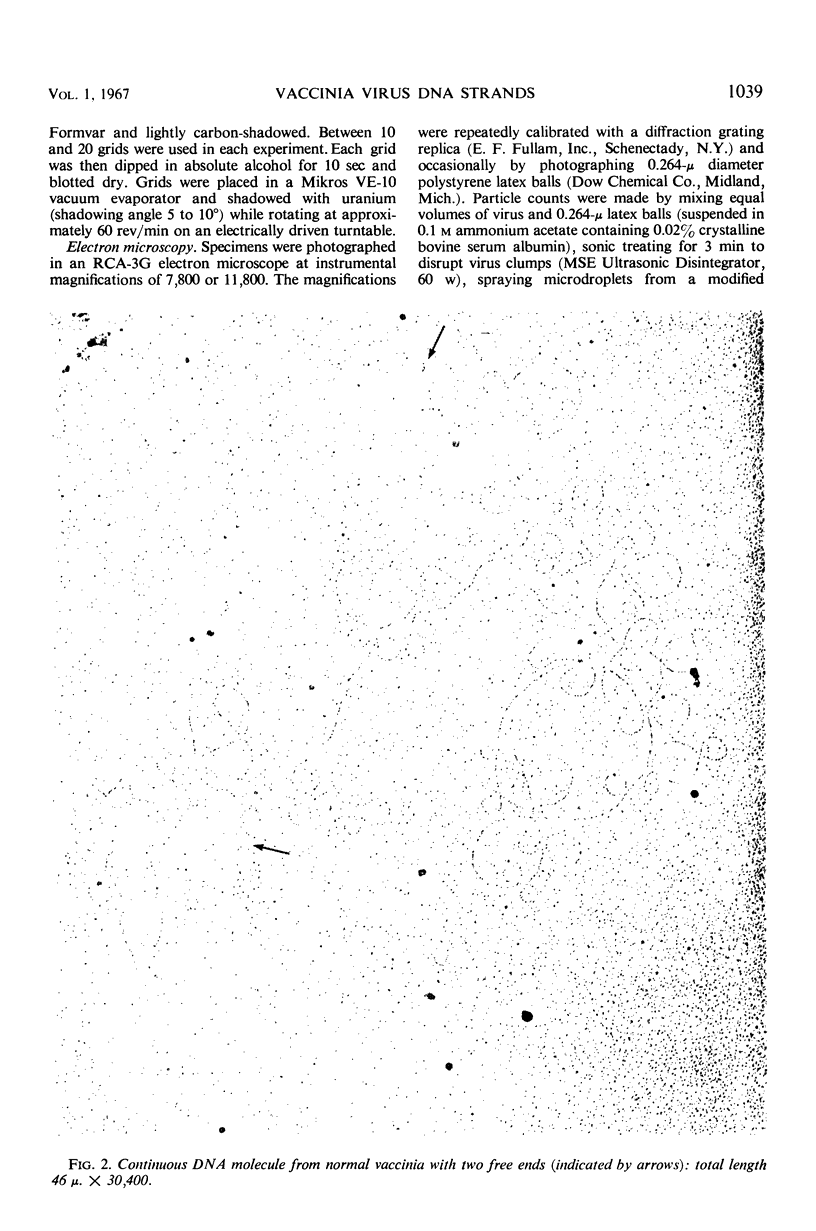
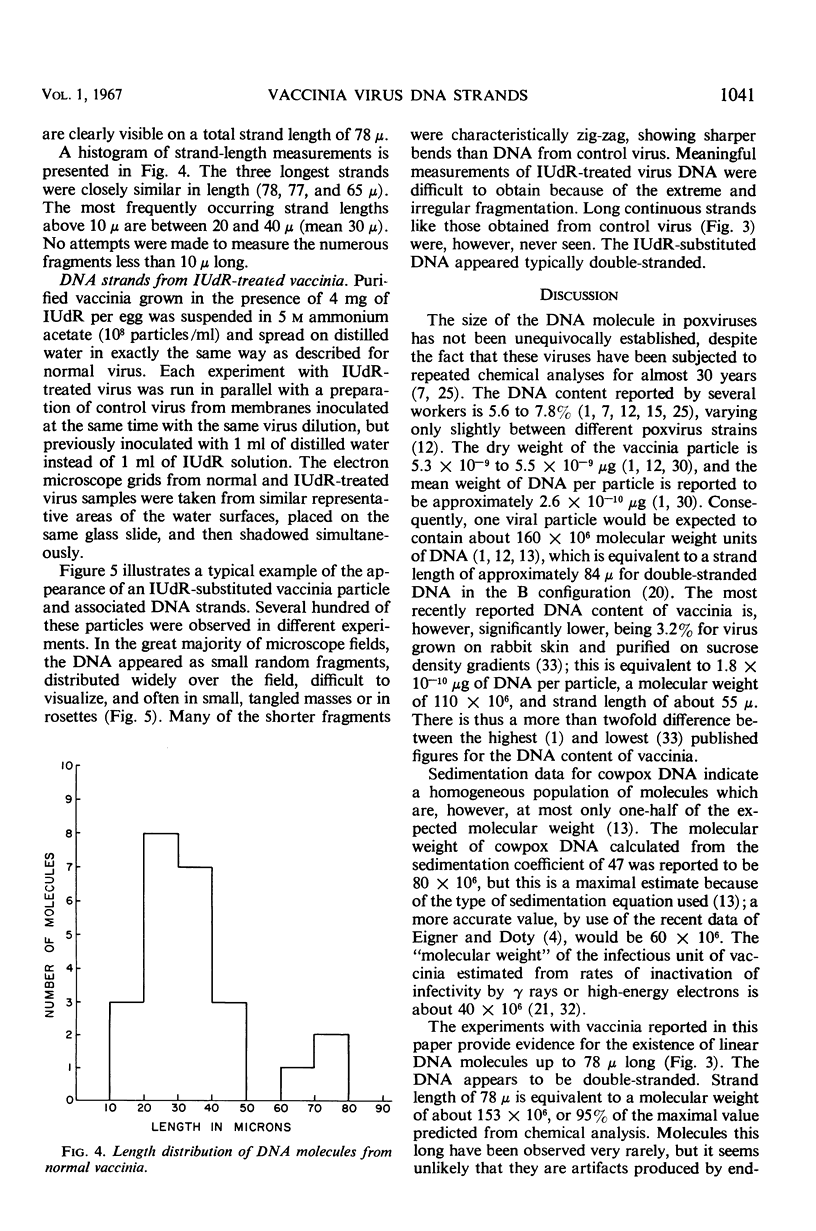
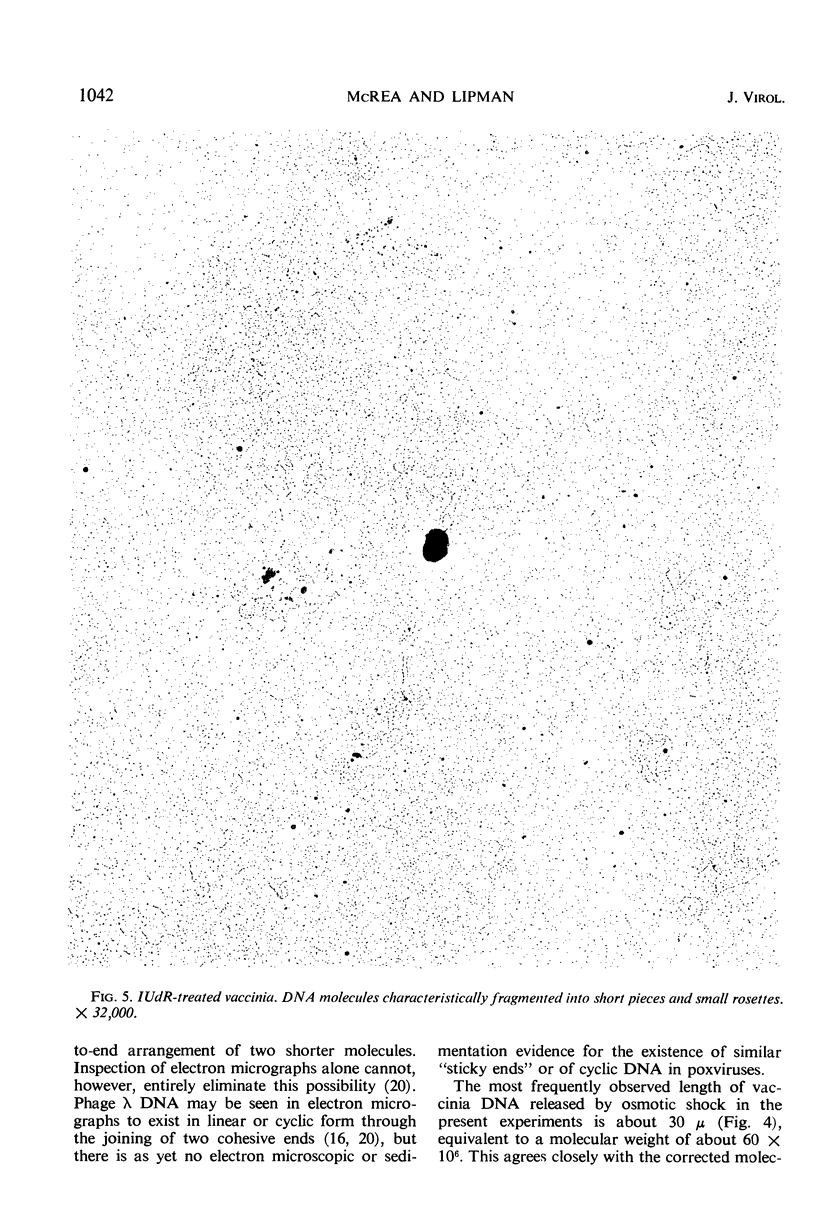
Purified vaccinia virus, which had been grown on chick-embryo chorioallantoic membranes in the presence or in the absence of 5-iodo-2′-deoxyuridine (IUdR), was suspended in 5 m ammonium acetate and subjected to the one-step Kleinschmidt procedure on surfaces of distilled water or salt solutions. Deoxyribonucleic acid (DNA) molecules were clearly revealed, and in many instances accurate length measurements could be made. The longest continuous molecules from normal virus measured 78, 77, and 65 μ. The most frequent length was approximately 30 μ, which corresponds to only one-third to one-half of the total DNA per virus particle predicted from various chemical analyses. These data provide direct evidence that normal vaccinia DNA may occur as a linear molecule of approximately 150 × 106 molecular weight units, but, for reasons still unknown, the majority of these molecules appears to break into segments of equal length during release from the virion. There is no evidence for the presence of cyclic DNA. The DNA molecules are typically double-stranded. DNA from IUdR-treated vaccinia presents a markedly different picture: the molecules are mostly fragmented into small pieces, and rosettes or tangled masses equivalent to even one-quarter the length of normal molecules occur very rarely. The possibility is discussed that at least part of the virus-inhibitory effect of IUdR on vaccinia is due to extensive fragmentation of the DNA molecules into which IUdR has been incorporated in place of thymidine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., BURKE D. C. The nucleic acid contents of viruses. J Gen Microbiol. 1962 Feb;27:181–194. doi: 10.1099/00221287-27-2-181. [DOI] [PubMed] [Google Scholar]
- CARO L. G. THE MOLECULAR WEIGHT OF LAMBDA DNA. Virology. 1965 Feb;25:226–236. doi: 10.1016/0042-6822(65)90201-1. [DOI] [PubMed] [Google Scholar]
- Eigner J., Doty P. The native, denatured and renatured states of deoxyribonucleic acid. J Mol Biol. 1965 Jul;12(3):549–580. doi: 10.1016/s0022-2836(65)80312-6. [DOI] [PubMed] [Google Scholar]
- GEMMELL A., FENNER F. Genetic studies with mammalian poxviruses. III. White (u) mutants of rabbitpox virus. Virology. 1960 May;11:219–235. doi: 10.1016/0042-6822(60)90063-5. [DOI] [PubMed] [Google Scholar]
- Hyde J. M., Randall C. C. Demonstration of deoxyribonucleic acid release from fowlpox virus. J Bacteriol. 1966 Mar;91(3):1363–1365. doi: 10.1128/jb.91.3.1363-1365.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INMAN R. B., SCHILDKRAUT C. L., KORNBERG A. ENZYMIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XX. ELECTRON MICROSCOPY OF PRODUCTS PRIMED BY NATIVE TEMPLATES. J Mol Biol. 1965 Feb;11:285–292. doi: 10.1016/s0022-2836(65)80058-4. [DOI] [PubMed] [Google Scholar]
- Inman R. B. A denaturation map of the lambda phage DNA molecule determined by electron microscopy. J Mol Biol. 1966 Jul;18(3):464–476. doi: 10.1016/s0022-2836(66)80037-2. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. I. THE FATE OF RADIOACTIVELY-LABELED RABBITPOX VIRUS. J Mol Biol. 1964 Feb;8:263–276. doi: 10.1016/s0022-2836(64)80136-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. The poxviruses. Bacteriol Rev. 1966 Mar;30(1):33–66. doi: 10.1128/br.30.1.33-66.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., LANG D., JACHERTS D., ZAHN R. K. [Preparation and length measurements of the total desoxyribonucleic acid content of T2 bacteriophages]. Biochim Biophys Acta. 1962 Dec 31;61:857–864. [PubMed] [Google Scholar]
- Kaiser A. D., Inman R. B. Cohesion and the biological activity of bacteriophage lambda DNA. J Mol Biol. 1965 Aug;13(1):78–91. doi: 10.1016/s0022-2836(65)80081-x. [DOI] [PubMed] [Google Scholar]
- Kaplan A. S., Benporat T., Kamiya T. Incorporation of 5-bromodeoxyuridine and 5-iododeoxyuridine into viral DNA and its effect on the infective process. Ann N Y Acad Sci. 1965 Jul 30;130(1):226–239. doi: 10.1111/j.1749-6632.1965.tb12556.x. [DOI] [PubMed] [Google Scholar]
- MACHATTIE L. A., THOMAS C. A., Jr DNA FROM BACTERIOPHAGE LAMBDA: MOLECULAR LENGTH AND CONFORMATION. Science. 1964 May 29;144(3622):1142–1144. doi: 10.1126/science.144.3622.1142. [DOI] [PubMed] [Google Scholar]
- OVERMAN J. R., TAMM I. Quantitative titration of vaccinia virus on the chorioallantoic membrane. J Immunol. 1956 Mar;76(3):228–236. [PubMed] [Google Scholar]
- PFAU C. J., MCCREA J. F. STUDIES ON THE DEOXYRIBONUCLEIC ACID OF VACCINIA VIRUS. III. CHARACTERIZATION OF DNA ISOLATED BY DIFFERENT METHODS AND ITS RELATION TO VIRUS STRUCTURE. Virology. 1963 Nov;21:425–435. doi: 10.1016/0042-6822(63)90204-6. [DOI] [PubMed] [Google Scholar]
- PFAU C. J., McCREA J. F. Release of deoxyribonucleic acid from vaccinia virus by 2-mercaptoethanol and pronase. Nature. 1962 Jun 2;194:894–895. doi: 10.1038/194894a0. [DOI] [PubMed] [Google Scholar]
- PRUSOFF W. H., BAKHLE Y. S., MCCREA J. F. INCORPORATION OF 5-IODO-2'-DEOXYURIDINE INTO THE DEOXYRIBONUCLEIC ACID OF VACCINIA VIRUS. Nature. 1963 Sep 28;199:1310–1311. doi: 10.1038/1991310a0. [DOI] [PubMed] [Google Scholar]
- Prusoff W. H., Bakhle Y. S., Sekely L. Cellular and antiviral effects of halogenated deoxyribonucleosides. Ann N Y Acad Sci. 1965 Jul 30;130(1):135–150. doi: 10.1111/j.1749-6632.1965.tb12548.x. [DOI] [PubMed] [Google Scholar]
- WEIL R., VINOGRAD J. THE CYCLIC HELIX AND CYCLIC COIL FORMS OF POLYOMA VIRAL DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:730–738. doi: 10.1073/pnas.50.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON D. E. Radiation inactivation of vaccinia virus. Radiat Res. 1961 Jun;14:796–802. [PubMed] [Google Scholar]
- ZWARTOUW H. T. THE CHEMICAL COMPOSITION OF VACCINIA VIRUS. J Gen Microbiol. 1964 Jan;34:115–123. doi: 10.1099/00221287-34-1-115. [DOI] [PubMed] [Google Scholar]






