Fig 1.
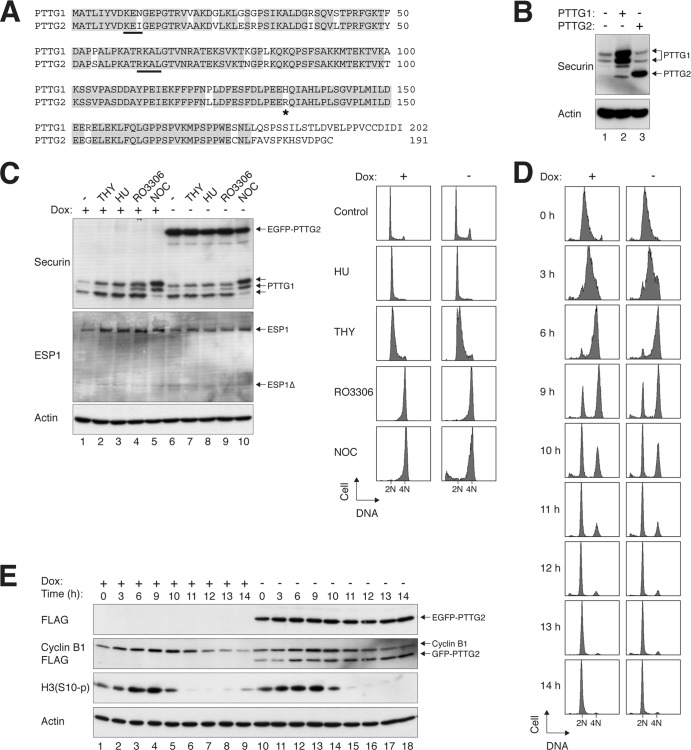
PTTG2 is a minor isoform of securin. (A) Sequence alignment of human PTTG1 and PTTG2. PTTG1 and PTTG2 share >80% identity at the amino acid level (>90% if the C terminus is excluded). Identical residues are highlighted. The destruction signals KEN box and D box (RXXL) of PTTG1 are underlined. The asterisk indicates the position of H134 in PTTG1 (or R134 in PTTG2). (B) PTTG2 is usually not present in HeLa cells. Lysates of asynchronously growing HeLa cells were prepared and analyzed with a PTTG1/2 monoclonal antibody (lane 1). Recombinant (untagged) PTTG1 and PTTG2 were expressed in HeLa cells and loaded side by side as standards. Note that PTTG1 appeared as a doublet and PTTG2 as a single band under these conditions. The overexpressed PTTG1 also contained some degradative products. (C) Ectopic expression of PTTG2 does not affect the cell cycle. Stable EGFP-PTTG2-expressing HeLa cells were cultured in the absence or presence of doxycycline (Dox) to turn on or off the expression of EGFP-PTTG2, respectively. The cells were exposed to thymidine (THY), hydroxyurea (HU), RO3306, or NOC for 16 h. Lysates were prepared and subjected to immunoblotting to detect the expression of endogenous PTTG1 and to confirm the expression of EGFP-PTTG2. Uniform loading of lysates was confirmed by immunoblotting for actin. The cells were also fixed, and the cell cycle profiles were analyzed by flow cytometry. (D) Cell cycle progression is not affected by PTTG2. HeLa cells that expressed FLAG-EGFP-tagged PTTG2 were generated. The cells were cultured continuously in the presence or absence of doxycycline to turn the PTTG2 off or on, respectively. After being synchronously released by a double-thymidine procedure, the cells were harvested at the indicated time points and analyzed by flow cytometry. The positions of 2N and 4N DNA contents are indicated. (E) Expression of mitotic markers is not affected by PTTG2. Cells were synchronized and harvested exactly as for panel D. Lysates were prepared and analyzed by immunoblotting. Equal loading of lysates was confirmed by immunoblotting for actin.