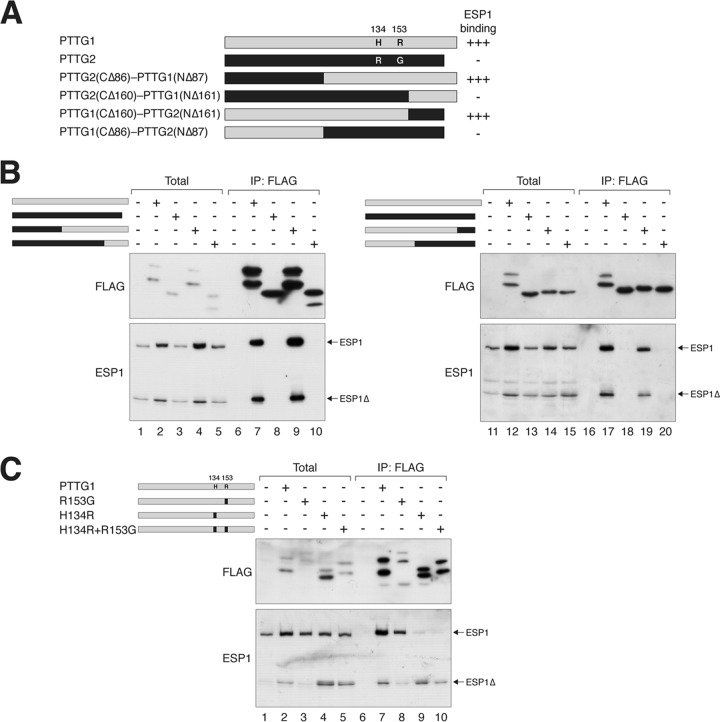
Fig 5.
H134 of PTTG1 is critical for securin function. (A) Chimeras between PTTG1 and PTTG2 reveal the importance of H134 or R153 for securin function. A schematic diagram of domain-swapping constructs between PTTG1 (gray) and PTTG2 (black) is shown to scale. A summary of the ESP1 binding is shown. Together with the data from the C-terminally deleted mutants, the region between 123 and 161 is likely to be critical for ESP1 binding. The positions and amino acids of the only 2 residues that are different between PTTG1 and PTTG2 within this region are indicated. (B) PTTG1-PTTG2 chimeras define a region critical for ESP1 binding. ESP1- and FLAG-tagged PTTG1-PTTG2 chimeras were expressed in HeLa cells as indicated (cf. panel A). Binding to ESP1 was detected by immunoprecipitation with FLAG antibodies, followed by elution and immunoblotting. (C) H134 of PTTG1 is crucial for ESP1 binding. H134 and R153 of PTTG1 (gray) were mutated to the corresponding sequence in PTTG2 (black) either individually or together. ESP1- and the indicated FLAG-tagged PTTG1 constructs were expressed in HeLa cells. Binding to ESP1 was assayed by immunoprecipitation with FLAG antibodies, followed by elution and immunoblotting. The expression of PTTG1R153G was lower in this experiment, which may explain the lower level of coimmunoprecipitated ESP1. There is no evidence from other experiments that PTTG1R153G binds ESP1 more weakly than wild-type PTTG1.