Fig 7.
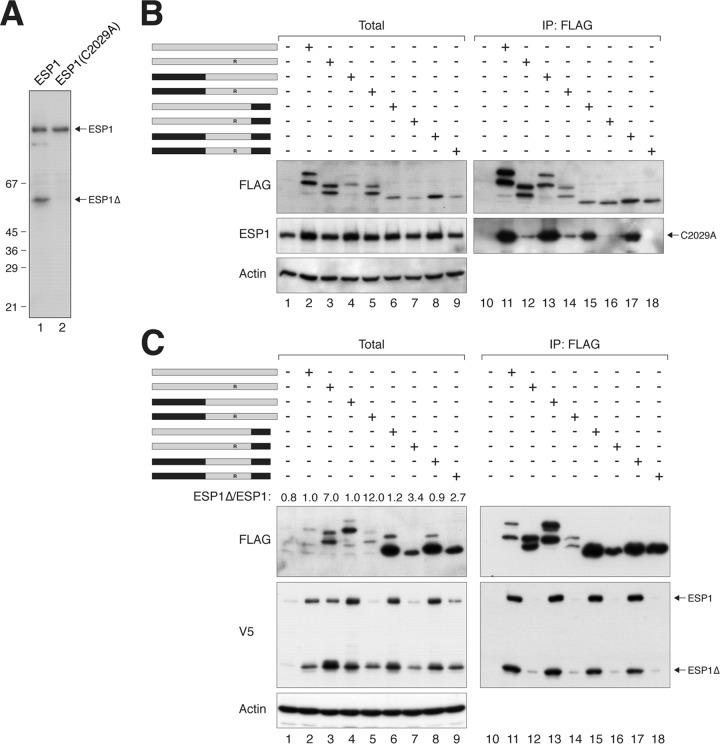
Evidence of an ESP1-activating domain in PTTG1. (A) ESP1C2029A abolishes autocleavage. HeLa cells were transfected with either wild-type ESP1 or catalytically inactive ESP1C2029A. Lysates were prepared and subjected to immunoblotting with ESP1 antibodies to detect the full-length and cleaved ESP1. The positions of molecular size standards (in kDa) are indicated. (B) PTTG1 does not strongly affect the expression of catalytically inactive ESP1. HeLa cells were cotransfected with plasmids expressing ESP1C2029A and FLAG-tagged PTTG1 or PTTG1-PTTG2 chimeras. A schematic diagram of the chimeras between PTTG1 (gray) and PTTG2 (black) is shown. Each pair of proteins consisted of a wild-type H134 and an H134R substitution. Lysates were prepared, subjected to immunoprecipitation with FLAG antibodies, and analyzed by immunoblotting. (C) The C-terminal region of PTTG1 is important for activation of ESP1. HeLa cells were cotransfected with plasmids expressing ESP1 and FLAG-tagged PTTG1 or PTTG1-PTTG2 chimeras. A schematic diagram of the chimeras between PTTG1 (gray) and PTTG2 (black) is shown. Each pair of proteins consisted of a wild-type H134 and an H134R substitution. Lysates were prepared, subjected to immunoprecipitation with FLAG antibodies, and analyzed by immunoblotting. The ratios between the cleaved ESP1 (ESP1Δ) and full-length ESP1 in the total lysates were quantified using serially diluted standards.