Fig 2.
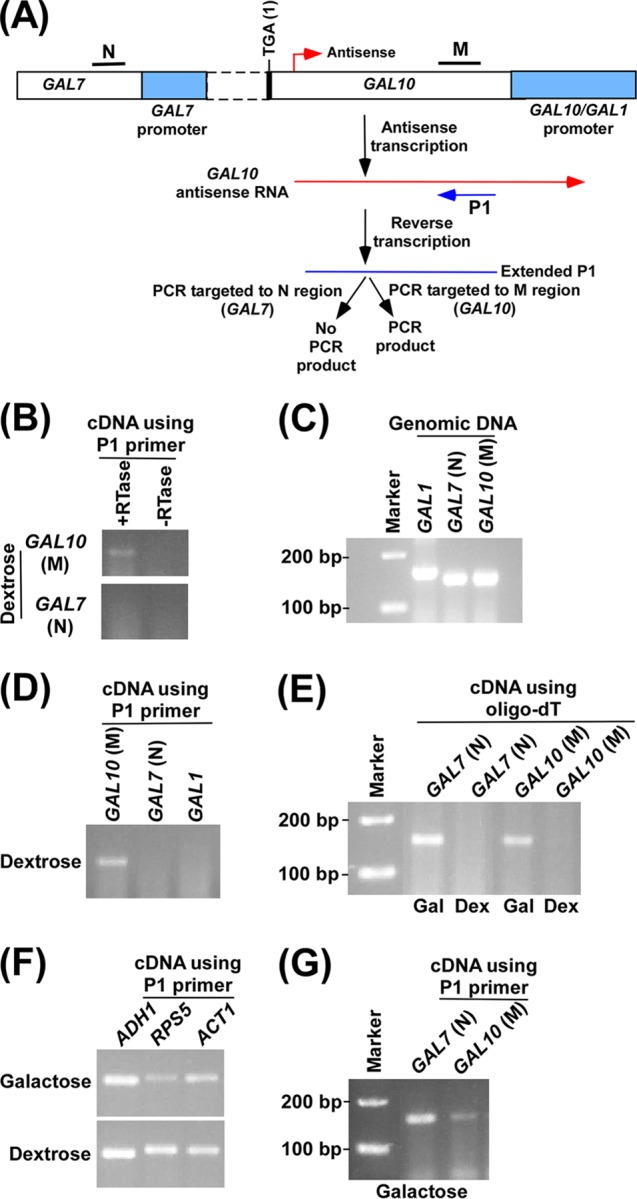
Analysis of GAL10 antisense transcript. (A) Schematic diagram showing the experimental strategy for the analysis of GAL10 antisense transcript. The P1 primer targeted toward the 5′ end of the GAL10 antisense transcript was extended by AMV reverse transcriptase-based reverse transcription at 42°C, and subsequently the extended primer was amplified by primer pairs targeted to the coding regions, M and N, of GAL10 and GAL7, respectively. (B) GAL10 antisense transcription in dextrose-containing growth medium. A yeast strain expressing Myc-tagged Rpb1p was grown in YPG medium to an OD600 of 0.8 at 30°C and then switched to YPD medium for 3 h. Total RNA was isolated and analyzed following the experimental strategy as described in panel A. The primer pair targeted to the GAL10 coding sequence (region M) generated PCR product from cDNA synthesized by the P1 primer. Reverse transcriptase was not used in cDNA synthesis in the −RTase lane. (C) Amplification of genomic DNA using PCR primer pairs targeted to the GAL7, GAL10, and GAL1 coding sequences. The same primer pairs were used as in panels A and B. (D) RT-PCR analysis in dextrose-containing growth medium as described in panel B. (E) RT-PCR analysis in dextrose-containing growth medium as described in panel B using oligo(dT) primer. A yeast strain expressing Myc-tagged Rpb1p was grown in YPG medium to an OD600 of 0.8 at 30°C and then transferred to YPG or YPD medium for 3 h. Gal, galactose; Dex, dextrose. (F) RT-PCR analysis as described in panel B in dextrose- and galactose-containing growth medium, using the P1 primer in cDNA synthesis. A yeast strain expressing Myc-tagged Rpb1p was grown as described in panel E. (G) RT-PCR analysis as described in panel B in galactose-containing growth medium.
