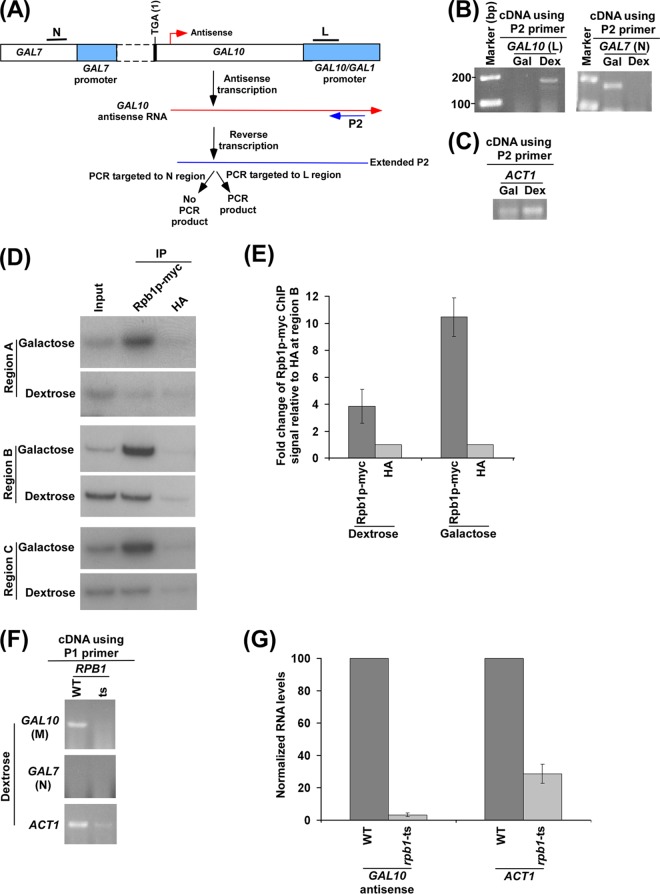
Fig 4.
RNA polymerase II is essential for GAL10 antisense transcription. (A) Schematic diagram showing the experimental strategy for the analysis of GAL10 antisense transcript using the P2 primer. The P2 primer targeted toward the 5′ end of the GAL10 antisense transcript, but encompassing a region corresponding to the GAL10-GAL1 bidirectional promoter, was extended by reverse transcription at 42°C, and subsequently the extended primer was amplified by primer pairs targeted to the core promoter region of GAL10 (region L) and the coding sequence region of GAL7 (region N). (B) GAL10 antisense transcription in dextrose-containing growth medium. The primer pair targeted to the GAL10 core promoter region (region L) but not the GAL7 coding sequence (region N) generated PCR product from cDNA synthesized by the P2 primer in dextrose-containing growth medium. A yeast strain expressing Myc-tagged Rpb1p was grown as described in the legend of Fig. 2E. (C) Amplification of cDNAs shown in panel B using a PCR primer pair targeted to the ACT1 coding sequences. (D) Analysis of RNA polymerase II at the 3′ end of the GAL10 coding sequence (region B) (Fig. 1A), GAL7 core promoter (region A) (Fig. 1A), and GAL10-GAL1 UAS (region C) (Fig. 1A) along with anti-HA as a nonspecific antibody control in dextrose- and galactose-containing growth media. A yeast strain expressing Myc-tagged Rpb1p was grown as described in the legend of Fig. 2E prior to cross-linking. Immunoprecipitations were performed as described in the legend of Fig. 1B. (E) The ratio of the ChIP signal of Myc-tagged Rpb1p to that of anti-HA (i.e., fold increase of Rpb1p-myc ChIP signal relative to HA) at region B in panel D is plotted in the form of a histogram. A ratio of 1 indicates no association of RNA polymerase II. (F) RNA polymerase II is essential for GAL10 antisense transcription in dextrose-containing growth medium. Wild-type (WT) and TS mutant strains of Rpb1p were grown in YPD medium at 23°C up to an OD600 of 0.85 and then switched to 37°C for 1 h prior to harvesting. Total RNA was prepared from the wild-type and TS mutant strains of Rpb1p and then analyzed for GAL10 antisense RNA using the P1 primer in cDNA synthesis. (G) The transcription data shown in panel F were plotted in the form of a histogram. The RNA level in the wild-type strain was set to 100, and the level of RNA in the mutant strain was normalized with respect to 100.