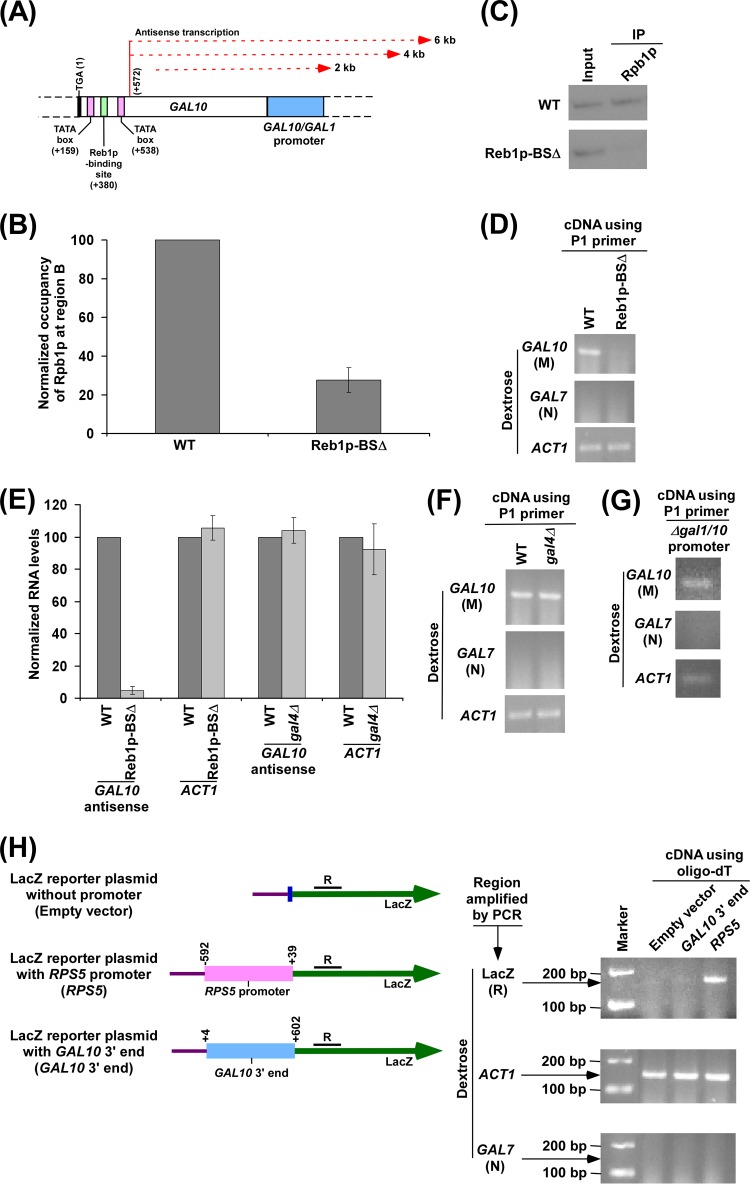
Fig 5.
Reb1p-binding site is essential for recruitment of RNA polymerase II at the 3′ end of the GAL10 coding sequence in dextrose-containing growth medium for GAL10 antisense transcription. (A) Schematic diagram showing the Reb1p-binding site, TATA box, and antisense transcription initiation site at the 3′ end of the GAL10 coding sequence. The numbers are presented with respect to the position of the translational stop codon of GAL10. (B) Analysis of antisense RNA polymerase II recruitment to the 3′ end of the GAL10 coding sequence in the wild-type (WT) strain and mutant carrying mutations in the Reb1p-binding site (Reb1p-BSΔ). Both the wild-type and mutant strains were grown as described in the legend of Fig. 1B. Immunoprecipitation was performed using 8WG16 antibody (Covance, Inc.) against the carboxy-terminal domain of the largest subunit (Rpb1p) of RNA polymerase II. Immunoprecipitated DNA was analyzed by PCR using a primer pair targeted to the 3′ end of the GAL10 coding sequence (region B) (Fig. 1A). The ChIP signal of the wild-type strain was set to 100, and the ChIP signal of the mutant strain was normalized with respect to 100 (represented as normalized or relative occupancy). (C) The autoradiograms for the ChIP data presented in panel B. (D) Analysis of GAL10 antisense transcription in the wild-type and Reb1p-BSΔ strains in dextrose-containing growth medium using the P1 primer in cDNA synthesis. Both wild-type and mutant strains were grown as described in panel B. (E) The transcription data in panels D and F were plotted in the form of a histogram. The PCR signal of GAL10 antisense transcript in the wild-type strain was set to 100, and the level of GAL10 antisense transcript in the mutant strain was normalized with respect to 100. Likewise, the ACT1 transcript level was set to 100 in the wild-type strain, and the level of ACT1 transcript in the mutant strain was normalized with respect to 100. (F) Analysis of GAL10 antisense transcription in the wild-type and Δgal4 strains in dextrose-containing growth medium. Both wild-type and mutant strains were grown as described in panel B. (G) Analysis of GAL10 antisense transcription in dextrose-containing growth medium in the yeast strain that does not have GAL1-GAL10 promoter. A yeast strain without a GAL1-GAL10 promoter was grown as described in panel B. (H) The GAL10 3′ end alone cannot drive transcription from a reporter plasmid. Schematic diagrams (left) show the LacZ reporter plasmid (pRS416) with RPS5 promoter or GAL10 3′ end. The numbers above RPS5 promoter (sense) are presented with respect to the first nucleotide of the transcription start site of RPS5. The numbers above GAL10 3′ end are presented with respect to the last nucleotide of the translational stop codon of GAL10. The region R at the LacZ coding sequence was amplified using cDNA generated by oligo(dT). Analysis of LacZ transcript under the RPS5 promoter or GAL10 3′ end was performed (right) The above constructs were transformed into wild-type yeast cells and then grown in dextrose-containing medium to an OD600 of 1.0 at 30°C prior to harvesting for RNA analysis. The coding regions of ACT1 and GAL7 were amplified as loading and no-DNA-contamination controls, respectively, using cDNA generated by oligo(dT).