Fig 1.
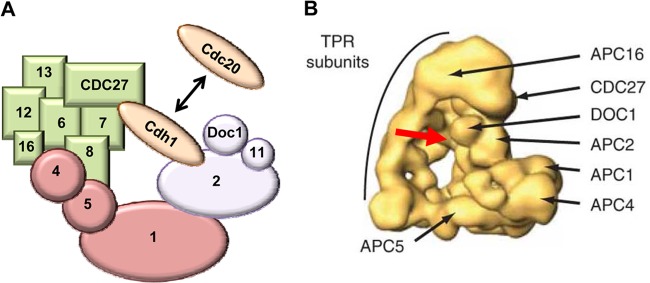
Structure of the APC. (A) Schematic structure of the APC. The APC is composed of three distinct subcomplexes that are color-coded for easy identification. The enzymatic unit (purple) contains the cullin and RING domains, which reside in APC2 and APC11, respectively. The specificity arm (also called the TPR subunits [green]) facilitates complex assembly as well as substrate binding. The bridge (pink) holds the other subcomplexes together. The coactivators (brown) bind to both the enzymatic unit and the specificity arm, where they interact with substrates. For clarity, the diagram is not drawn to scale and not all subunits are shown. (B) The human APC structure derived from single-particle electronic microscopic analysis and three-dimensional (3D) reconstruction (73). Shown are the triangular architecture of the APC and the locations of representative subunits of the specificity arm (i.e., APC3/CDC27, APC16, and other TPR subunits), enzymatic unit (i.e., APC2 and APC10/Doc1), and bridge (i.e., APC1, APC4, and APC5). The red arrow depicts the binding site of the APC coactivator (e.g., Cdh1, which is not shown in the image).
