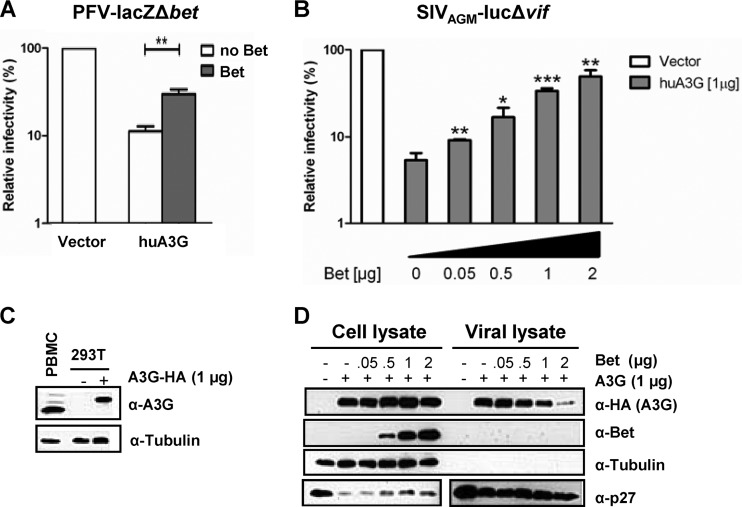
Fig 1.
Bet counteracts A3G in a dose-dependent manner and independent of the virus background. (A) Bet-deficient lacZ reporter viruses derived from PFV were produced in the presence and absence of human APOBEC3G (huA3G) and Bet. Infectivities of viruses were determined by quantification of galactosidase expression at 3 dpi. Infectivities relative to that of the virus generated in the absence of huA3G and Bet are given. (B) SIVAGM-lucΔvif viruses were produced in the presence and absence of huA3G and increasing amounts of Bet. Infectivities of equal amounts of viruses, relative to that of the virus generated in the absence of huA3G and Bet, were determined by quantification of luciferase activity at 3 dpi. (C) Endogenous level of A3G in activated human PBMCs compared to plasmid-derived A3G level in transfected 293T cells (1 μg A3G-HA plasmid), detected by using anti-A3G antiserum. Equal loading of cell lysate samples was confirmed with an anti-tubulin antibody. (D) Packaging of A3G in SIVAGM-lucΔvif virions. Virions were produced in the presence of an empty expression plasmid (−; pcDNA3.1) or 1 μg A3G expression plasmid and increasing amounts of Bet expression plasmid (0 μg, 0.05 μg, 0.5 μg, 1 μg, and 2 μg). Supernatants were filtered and concentrated through a 20% sucrose cushion by centrifugation. The cell and viral lysates were analyzed by immunoblotting and were probed for A3G (α-HA, anti-HA antibody), Bet (α-Bet, anti-Bet antibody), capsid (α-p27, anti-p27 antibody), and tubulin (α-Tubulin, anti-tubulin antibody). Unpaired t tests were computed to determine whether differences between samples in the presence of A3G protein with and without Bet reached the level of statistical significance (*, P < 0.05; **, P < 0.001; and ***, P < 0.0001), using GraphPad Prism 5 software.