Fig 6.
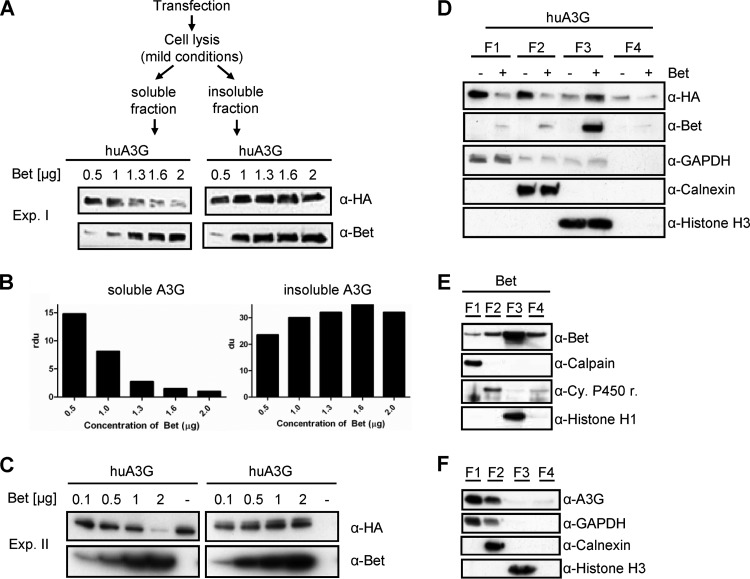
Bet impairs the cytosolic solubility and modifies the buffer-dependent detection of huA3G. (A) 293T cells transfected with huA3G and increasing amounts of Bet expression plasmid (0.5, 1, 1.3, 1.6, and 2 μg; Exp. I) were lysed under mild conditions. Soluble and insoluble fractions were separated by centrifugation and analyzed by SDS electrophoresis and subsequent immunoblotting. HA-tagged A3G was detected with an anti-HA MAb, and Bet was detected with Bet antiserum. (B) Relative density units (rdu) of A3G signals shown in panel A. (C) Independent experiment II (Exp. II) was performed as described for panel A, except that 0.1, 0.5, 1, and 2 μg of Bet expression plasmid were used. (D) Differential detergent fractionation of proteins of 293T cells transfected with expression plasmids for A3G-HA, with and without Bet, according to their solubilities in buffers specific for the following subcellular fractions: cytoplasm (F1; digitonin extraction), membranes and organelles (F2; Triton X-100 extraction), nucleus (F3; Tween20/deoxycholate extraction), and cytoskeleton (F4; SDS extraction). HA-tagged A3G on immunoblots was detected with an anti-HA MAb, and Bet was detected with Bet antiserum. Equal loading of samples and proper fractionations were confirmed with an anti-GAPDH MAb for cytosolic fractions, anti-calnexin MAb for membrane/organelle fractions, and anti-histone H3 MAb for nuclear fractions. (E) Immunoblot analysis of Bet without huA3G in different fractions of 293T cells. The F1 fraction was detected using an anti-calpain MAb, the F2 fraction using an anti-cytochrome 450 reductase MAb, and the F3 fraction using an anti-histone H1 MAb. (F) Immunoblot analysis of endogenous levels of huA3G in different fractions of activated human peripheral blood mononuclear cells, using huA3G antiserum.