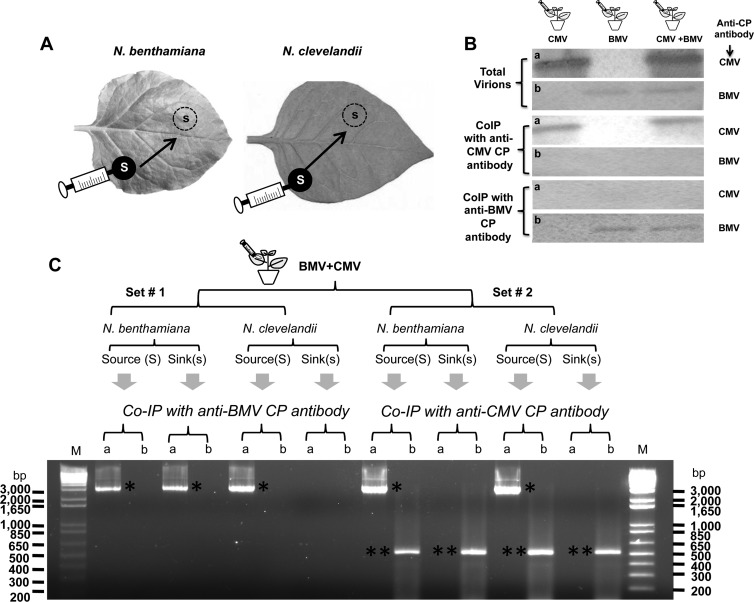
Fig 8.
Coexpression of BMV and CMV in N. benthamiana and N. clevelandii. (A) Cultures of agrotranformants containing a full complement of wt BMV or wt CMV, or both, were allowed to spot infiltrate N. benthamiana or N. clevelandii as discrete spots referred to as the source (S; filled circles), and the progeny was analyzed from the sink (s; dotted circles). (B) Western blot analysis of BMV and CMV virions from coinfiltrated leaves by co-IP as described in Materials and Methods. Total virions purified from leaves infiltrated with BMV or CMV, or both viruses, were subjected to co-IP with either anti-CMV CP antibody or anti-BMV CP antibody. Precipitated virions were subjected to Western blot analysis by probing with either anti-CMV CP antibody (a) or anti-BMV CP antibody (b). (C) trans-Encapsidation assays. Leaves of N. benthamiana and N. clevelandii coexpressing BMV and CMV were divided into two sets (set 1 and set 2), as shown schematically. Virions purified from leaf discs about 15 mm in diameter excised from the source and sink areas were subjected to co-IP with either anti-BMV CP antibody or anti-CMV CP antibody. Virion RNA products isolated by co-IP were subjected to RT-PCR using primers designed to specifically amplify either the 3.2-kb full-length fragment of RNA 1 of BMV (a) or a 500-nt fragment encompassing the CMV RNA 1 sequence (b) (see Materials and Methods). The resulting PCR products were analyzed by agarose gel electrophoresis, followed by ethidium bromide staining. DNA size markers (lanes M) are shown to right and left of the gel. Single and double asterisks, electrophoretic mobility of PCR products corresponding to BMV RNA 1 and CMV RNA 1, respectively.