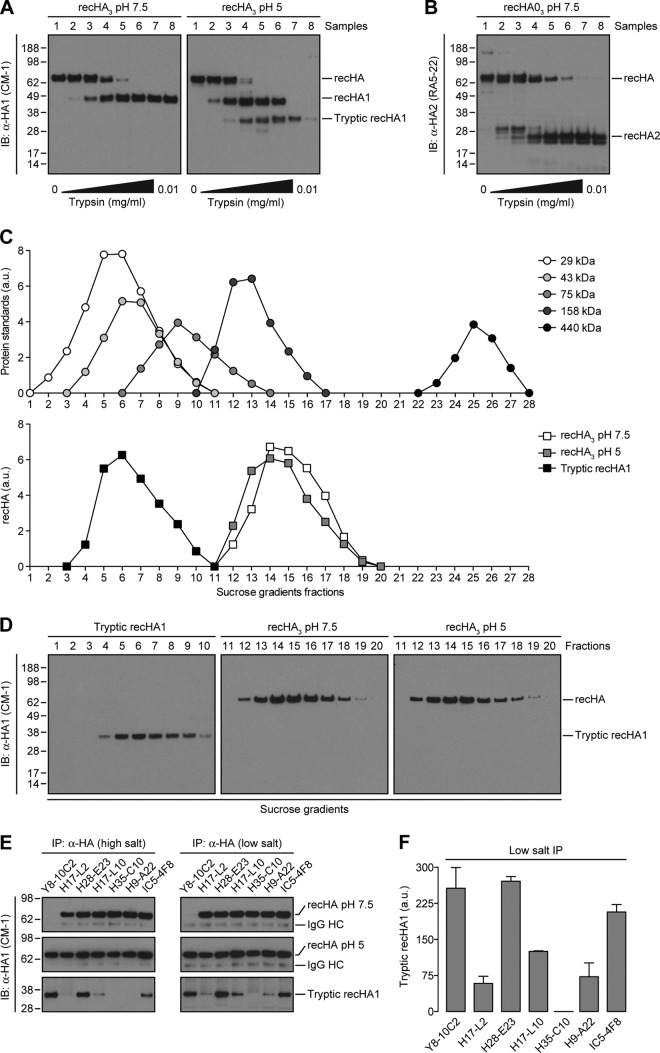
Fig 5.
HA trimer-specific MAbs to the Ca, Cb, and Sa antigenic sites bind to monomers derived from oligomerized HA. (A and B) Untreated (pH 7.5) and acid-triggered (pH 5) recHA3 were incubated with increasing amounts of trypsin for 15 min at 37°C. Digestion was stopped with 1 mM PMSF. recHA1- (A) or recHA2 (B)-derived fragments were then visualized by immunoblotting (IB) with the anti-HA CM-1 or RA5-22 MAbs, respectively. (C and D) In addition to a cocktail of protein standards, native recHA3, acid-treated recHA3, and the monomeric, tryptic fragments derived from recHA1 (HA tops) (sample 7, A) were fractionated by ultracentrifugation on layered 5 to 25% sucrose gradients as described in Materials and Methods. Sucrose gradient fractions were then analyzed by reducing SDS-PAGE and immunoblotting with the CM-1 MAb (D). The amounts of sedimented standards and recHA proteins in each fraction were quantified by densitometry and expressed in arbitrary units (a.u.) (C). (E) The recHA3 pH 7.5, recHA3 pH 5, and monomeric tryptic recHA1 proteins were subjected to IP at a high (300 mM) or low (150 mM) salt concentration with the HA monomer-specific Y8-10C2 MAb; the HA trimer-reactive H17-L2, H17L-10, H35-C10, and H9-A22 MAbs; or MAbs H28-E23 and IC5-4F8, which recognize both HA species. (F) The amount of immunoprecipitated HA tops was quantified as described in the legend to Fig. 1D. The values to the left of panels A, B, D, and E are molecular sizes in kilodaltons.