Fig 4.
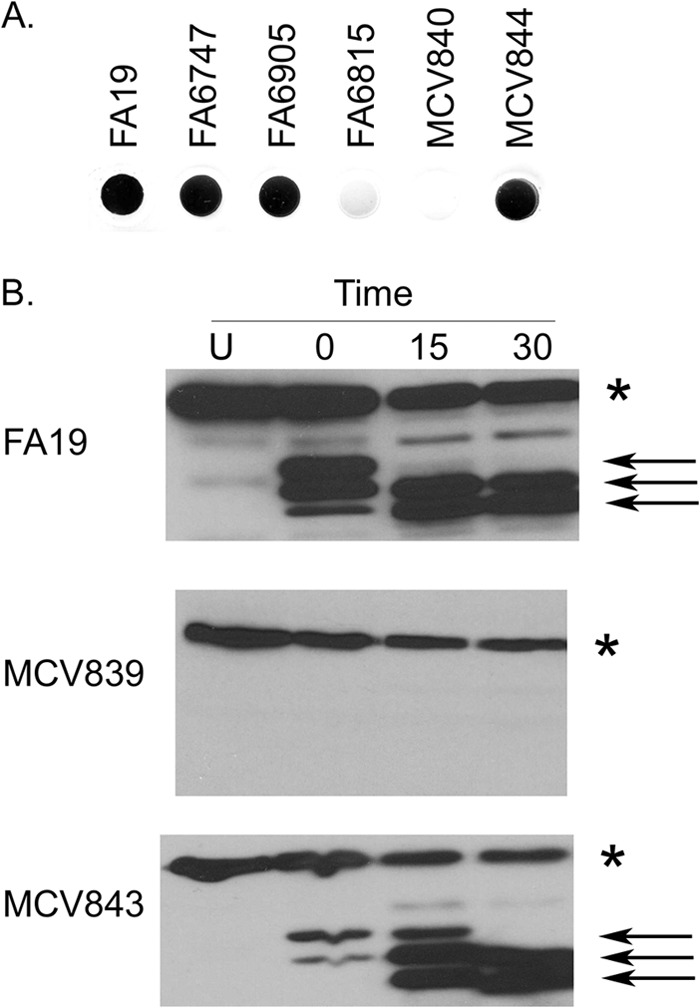
TbpB LSAC is retained on the cell surface only when TbpA is also produced. (A) Bacteria were grown under iron-depleted conditions and spotted onto nitrocellulose. Blots were probed with hTf-HRP. Strains tested included the controls FA19 (wild type), FA6747 (TbpA−), FA6905 (TbpB−), and FA6815 (TbpA− TbpB−). MCV840 produces TbpB LSAC in the background of a strain that does not produce TbpA. MCV844 produces TbpB ΔGly in the background of a strain that does not produce TbpA. (B) Bacteria were grown under iron-depleted conditions prior to exposure to trypsin. Whole cells were exposed to trypsin for 0, 15, or 30 min, after which aprotinin was added to stop digestion. Undigested samples (U) were also included as a control. Whole-cell lysates were generated from trypsin-treated cells and controls. Proteins were separated by SDS-PAGE, transferred to nitrocellulose, and probed with hTf-HRP. Strains tested include MCV839 (produces the TbpB LSAC mutant in the background of a strain that produces TbpA), MCV843 (produces the TbpB ΔGly mutant in the background of a strain that produces TbpA), and the wild-type strain, FA19. The asterisk marks the location of full-length TbpB. TbpB produced by all of the strains is degraded over time of exposure to trypsin, indicating that TbpB is surface exposed in these strains. The arrows mark the positions of lipidated, proteolytically cleaved TbpB fragments, which remain tethered to the cell surface.
