Fig 3.
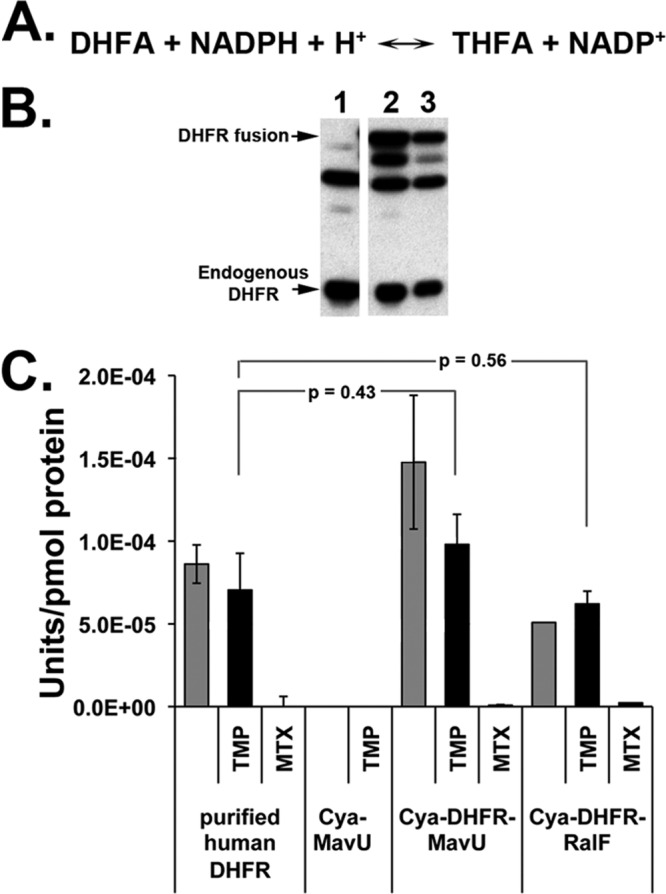
The DHFR moiety within the DHFR-IDTS fusions is catalytically active. (A) NADPH-dependent reduction of dihydrofolic acid (DHFA) to tetrahydrofolic acid (THFA) catalyzed by DHFR. (B) Immunoblot of L. pneumophila soluble extracts from strains overexpressing CyaA-MavU (lane 1) or two different cultures of CyaA-DHFR-MavU proteins (lanes 2 and 3). Antibody against DHFR was used to determine expression of the CyaA-DHFR-IDTS or endogenous DHFR. Densitometry of the CyaA-DHFR-IDTS bands allowed for quantification of protein concentration for determining specific activity. (C) DHFR fusion proteins are enzymatically active. Bacterial cultures were grown in the presence of IPTG to induce expression of the indicated protein fusions. Cells were lysed, and the soluble protein extracts were combined with 100 μM NADPH, 100 μM dihydrofolic acid, and either 50 μM TMP or MTX to inhibit endogenous bacterial DHFR activity or all DHFR activity, respectively. Purified human DHFR was used as a positive control. Activity was normalized to DHFR protein levels as determined by Western blotting. P values are determined by Student's t test, comparing DHFR activity of fusions in the presence of TMP to the purified human DHFR control.
