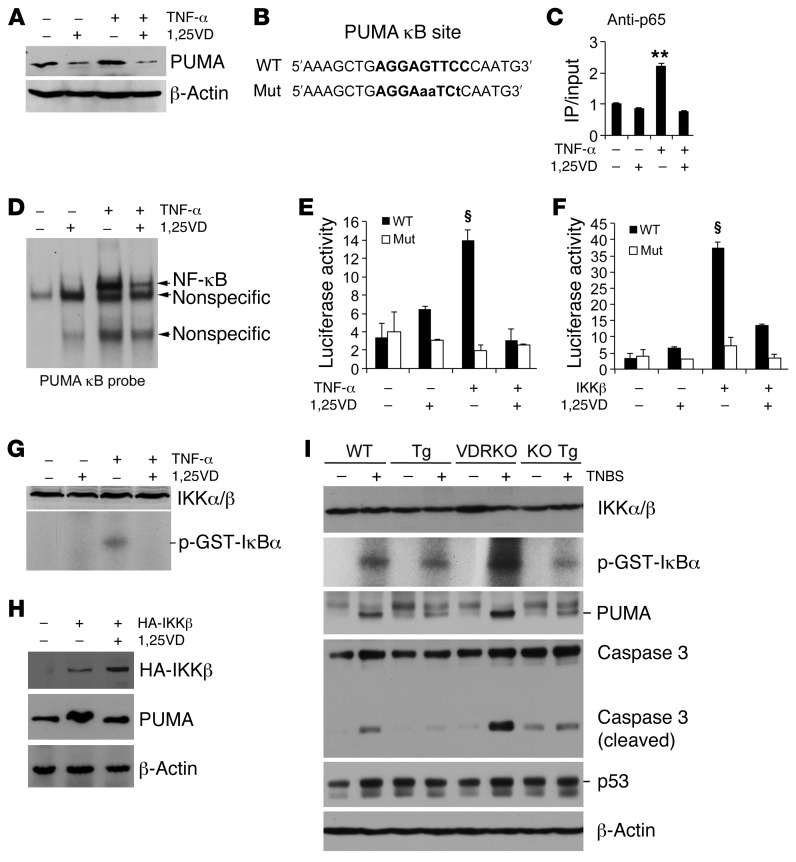
Figure 9. Epithelial VDR signaling abrogates PUMA induction by blocking NF-κB activation.
(A) Western analysis of PUMA in HCT116 cells treated with TNF-α (100 ng/ml) ± 1,25(OH)2D3 (1,25VD, 20 nM). (B) PUMA gene promoter κB cis-element and its mutant sequences. (C) ChIP assay measuring p65 binding to the κB site in HCT116 cells treated with TNF-α ± 1,25(OH)2D3. **P < 0.01 versus the rest. (D) EMSA using 32P-labeled PUMA κB probe and nuclear extracts isolated from HCT116 cells treated with TNF-α ± 1,25(OH)2D3. (E) PUMA promoter luciferase reporter assays in HCT116 cells transfected with wild-type (WT) or mutant (Mut) PUMA κB luciferase reporter, followed by treatment with TNF-α ± 1,25(OH)2D3. (F) HCT116 cells were cotransfected with the WT or Mut PUMA κB luciferase reporter and IKKβ-expressing plasmid. Luciferase activity was determined after 1,25(OH)2D3 (+) or ethanol (–) treatment. §P < 0.001 versus the rest. (G) IKK kinase assays in HCT116 cells treated with TNF-α ± 1,25(OH)2D3. (H) HCT116 cells were transfected with empty vector (–) or HA-IKKβ plasmid (+), followed by treatment with ethanol (–) or 1,25(OH)2D3 (+). Note that PUMA protein was induced by IKKβ overexpression, and this induction was abolished by 1,25(OH)2D3. (I) Colonic mucosal IKK activity. Colonic mucosa were isolated from untreated (–) and TNBS-treated (+) WT, Tg, VDRKO and KO Tg mice on day 2 after TNBS treatment, and the lysates were subjected to IKK kinase assays and Western analyses for IKKα/β, PUMA, caspase 3, and p53 proteins. Each lane represents a pool of 4 to 5 mice of the same genotype and treatment.