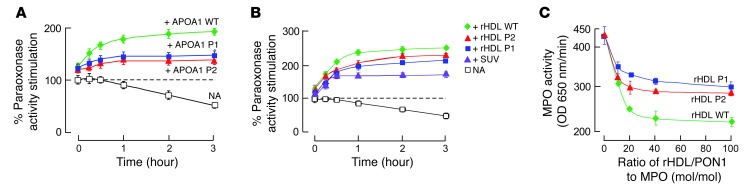
Figure 4. Demonstration that APOA1 P1 and P2 regions are functionally important in the HDL-PON1-MPO ternary complex.
(A) APOA1 harboring mutations within P1 or P2 region (versus WT) were incubated with PON1 at 37°C for the indicated times, and then paraoxonase activity was determined. Results were normalized (100%) to PON1 activity measured with no addition (NA) at t = 0. (B) PON1 was incubated with reconstituted HDL (rHDL; 100:10:1, OPC/cholesterol/APOA1, mol/mol/mol) using the indicated APOA1 forms (WT versus P1 versus P2 mutants) or small unilamellar vesicles (SUV) composed of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) (16:0, 18:1 phosphatidylcholine [PC]) for the indicated times, and then paraoxonase activity was measured. Results were normalized (100%) to PON1 activity measured with no addition at t = 0. (C) TMB assay showed the effect of varying levels of the binary complex composed of PON1 and the indicated rHDL (made with WT versus P1 versus the P2 APOA1 mutant) on MPO activity. All results represent the mean ± SD from at least 3 independent experiments. In A and B, the differences between WT and mutants at the 3-hour point were significant (APOA1 WT versus APOA1 P1, P < 0.05; APOA1 WT versus APOA1 P2, P < 0.05). In C, the difference between WT and mutants at a molar ratio of rHDL/PON1 to MPO of 20 or greater were significant (rHDL WT versus rHDL P1, P < 0.05; rHDL WT versus rHDL P2, P < 0.05).