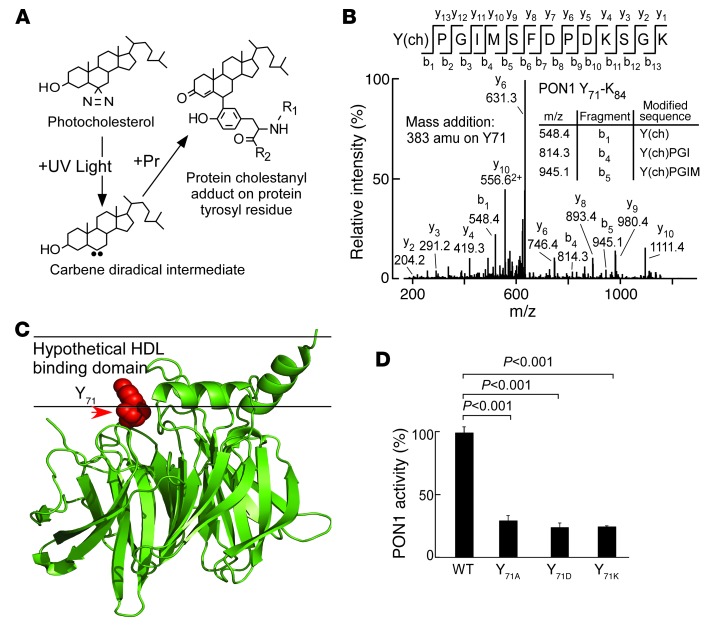
Figure 5. PON1 Tyr71 is a functionally important site for HDL interaction and a target for MPO-catalyzed oxidation in human atherosclerotic plaque.
(A) Schematic illustration of photocholestanyl-labeled PON1 tyrosyl residue. (B) Reconstituted HDL formed using POPC/photocholesterol/APOA1 (100:10:1) was incubated with PON1 and exposed to UV light as described in Methods. Proteomics studies revealed the photocholestanyl adduct of PON1 Tyr71 [Y(ch)] as a site on PON1 that directly interacts with cholesterol as described in Methods. m/z, mass-to-charge ratio. (C) PON1 crystal structure with superimposed location of Tyr71 (red) and hypothetical HDL binding surface based on hydrophobicity analysis. (D) Recombinant human WT PON1 versus the indicated PON1 Tyr71 site–specific mutants were generated, isolated, incubated with HDL, and then paraoxonase activity was determined as described in Methods. Results represent the mean ± SD from at least 3 independent experiments.