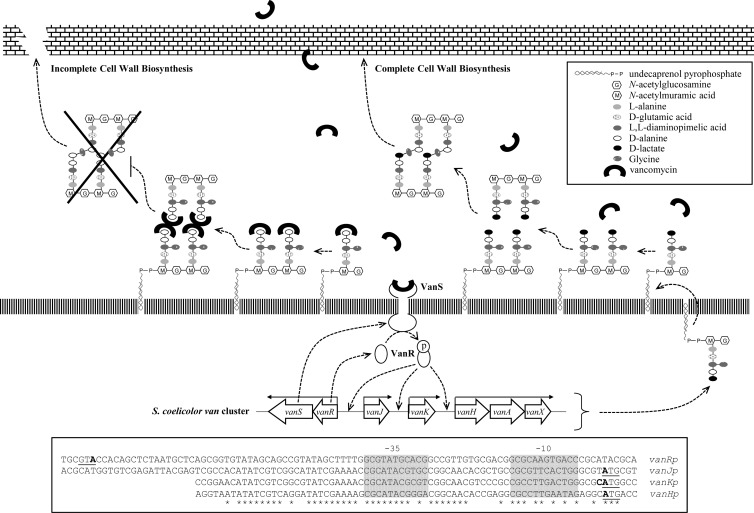
Fig 1.
Model illustrating the mode of action of vancomycin and the vancomycin resistance mechanism in S. coelicolor. Vancomycin binds to the d-Ala-d-Ala dipeptide termini of non-cross-linked peptidoglycan pentapeptide side chains, preventing the formation of mature peptidoglycan and lowering its strength. Resistance is initiated when vancomycin is directly perceived by the sensor kinase, VanS, which then phosphorylates its cognate partner response regulator, VanR. Phospho-VanR activates the expression of the van gene cluster and results in the replacement of the d-Ala-d-Ala dipeptide terminus of cell wall PG precursors by d-Ala-d-Lac, which significantly reduces the affinity of vancomycin binding by ∼1,000-fold, consequently rendering the modified bacteria resistance to vancomycin. The boxed area presents the promoter sequences upstream of each transcription unit with the ATG translational start sites underlined and the −10 and −35 consensus sequences indicated. Transcription start sites are written in bold, and highly conserved bases are marked with asterisks.