Fig 6.
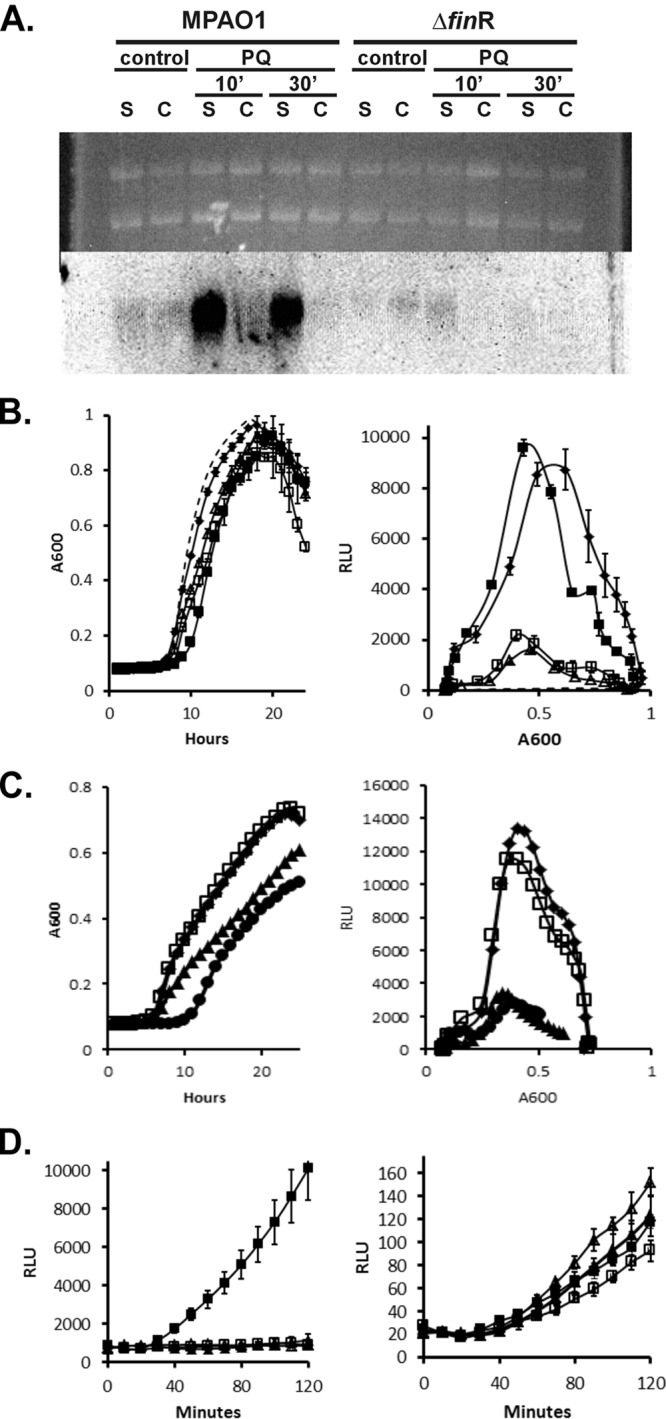
P. aeruginosa shows finR-dependent fprA expression which is responsive to reduced sulfur sources. (A) Northern analysis of cultures of wild type (MPA01) or finR deletion mutant (Δ3398) grown with sulfate as the sole sulfur source (S), or sulfate plus l-cystine (C). Paraquat-treated cultures (PQ) were exposed to 1 mM paraquat for 10 or 30 min with the respective sulfur supplements prior to RNA extraction. Upper portion, ethidium-stained gel; lower portion, Northern blot hybridized with an fprAPa probe. A total of 10 μg of RNA was loaded per lane. (B) Complementation analysis of the finR deletion strain Δ3398. Cultures were grown with sulfate as the sole sulfur source. The expression of a chromosomal luxCDABE operon fused to the PfprAPa promoter was monitored by luminescence. Left, growth; right, luminescence versus cell density (RLU, relative luminescence units). Symbols: ◆, MPA01; ▲, Δ3398; □, Δ3398/pMF54 (vector control); ■, 3398/pMF418 (plasmid-borne finRPa); dashed line, MPA01::Tn7lux control (no promoter). (C) Expression of PfprA-lux reporter by wild-type P. aeruginosa during growth with various sole sulfur sources. Symbols: ◆, sulfate; □, sulfite; ▲, cystine; ●, cysteine. (D) Expression of PfprA-lux reporter by wild type and ΔfinR mutant strains in response to paraquat and/or cysteine. Exponential-phase cells grown on minimal medium with sulfate as sulfur source were diluted into the same medium with or without additions of 1 mM paraquat or 0.5 mM cysteine. Left, wild type (MPA01); right, ΔfinR. Symbols: □, no addition; ■, 1 mM paraquat; △, cysteine; ▲, cysteine plus 1 mM paraquat.
