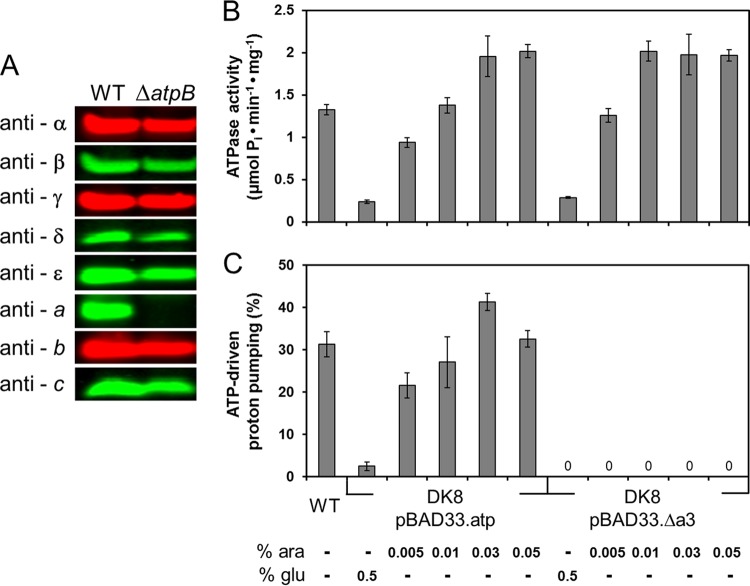
Fig 1.
Characterization of the FOF1-a subcomplex. (A) Cells of E. coli DK8 transformed with pBWU13 (WT) or pJGA1 (ΔatpB) were grown in LB medium with ampicillin and harvested at OD = 0.8 to 1.0. After resuspension in sample loading buffer, cells were incubated for 5 min at 99°C. The amount of cell extract applied per lane (20 μg) was calculated according to the determination of Neidhardt et al. (38) that 160 μg of protein was present per ml cell culture at OD = 1.0. Immunolabeling was performed using mouse (anti-β, anti-δ, anti-ε) or rabbit (anti-α, anti-γ, anti-b) polyclonal antisera or mouse monoclonal antibodies (anti-a, anti-c) raised against the individual subunits of the ATP synthase. (B and C) ATP hydrolysis (B) and ATP-driven proton-pumping activity (C) of membrane vesicles of DK8/pBAD33.atp and DK8/pBAD33.Δa3, respectively, determined using the WT as a control. Cells were grown in LB medium with chloramphenicol in the presence of different arabinose concentrations for induction of the araBADp expression system or in the presence of glucose for repression as indicated. Cells were harvested at OD = 0.8 to 1.0, and inverted membrane vesicles were prepared. ATP-driven proton translocation was measured via ACMA fluorescence quenching. The relative magnitudes of quenching induced by addition of ATP are shown. ara, arabinose; glu, glucose.